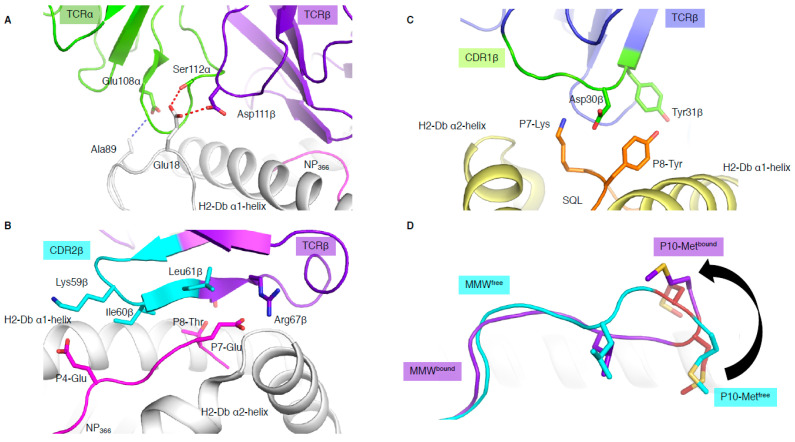
Figure 3.
Unconventional TCR docking or TCR–peptide interactions. (A) NP2-B17 TCR (α in green and β in purple) contacting the loops outside the H-2Db antigen-binding cleft, and, namely, the residues 18 and 89 (white stick). The red and blue dashed lines represent the hydrogen and Van der Waals interactions. (B) The NP2-B17 TCR CDR2β (cyan) and framework β (purple) interact with the NP366 peptide (pink) presented by H-2Db (white). (C) TRBV13-3+ TCR (β chain in blue) with the CDR1β (green) residues (sticks) interacting specifically with the malaria-derived SQL peptide (orange) P7-Lys and P8-Tyr (sticks) presented by the H-2Db (yellow). (D) Comparison of the MMW peptide structure (loop) presented by human leukocyte antigen (HLA)-A*02:01 without (cyan) or with the DMF5 TCR (purple). The DMF5 TCR binding leads to a register shift of the MMW peptide, whereby the P10-Metfree in the HLA cleft (cyan sticks) is flipped out of the cleft (P10-Metbound in purple stick) upon binding of the DMF5 TCR.