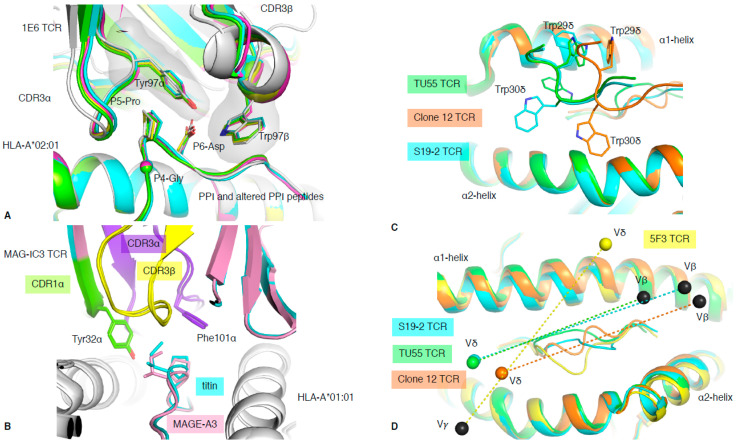
Figure 5.
TCR cross-reactivity and non-αβ TCR recognition. (A) Structural overlay of the 1E6 TCR in complex with the PPI (white) and altered PPI peptides: YQF (green), RQW (orange), MVW (cyan), RQF-I (pink), and RQF-A (yellow). The conserved 4GPD6 motif in the peptide is represented as sticks and spheres for the Cα atom of the P4-Gly. The CDR3 loop residue Tyr97α and Trp97β forming the hydrophobic cap are represented as sticks. (B) Structural overlay of the MAG-IC3 TCR in complex with the HLA-A*01:01 (white) presenting the MAGE-A3 (pink) or titin (cyan) peptide. The TCR is coloured as per the bound peptide, with the CDR1α in green, CDR3α in purple, and CDR3β in yellow. (C) Top view of structural overlay of δβ TCRs in complex with pHLA-I complex. The TU55 TCR-HLA-B*35:01-IPL is in green, S19-2 TCR-HLA-A24:02-RYP is in cyan, and clone 12 TCR-HLA-B*35:01-IPS is in orange. The conserved TRDV1*01 CDR1δ is represented as a loop with the Trp29δ and Trp30δ represented as sticks. (D) As per panel (C), the top view of the MHC-I cleft with a structural overlay of three δβ TCRs–pMHC-I complex and the 5F3 γδ TCR added (yellow). The mass centres for the Vβ or Vγ are represented as black spheres, while the Vδ mass centres are presented as coloured spheres matching each TCR as per panels (C,D). All the structural overlays are aligned on the MHC-I cleft (residues 1–180).