Figure 1.
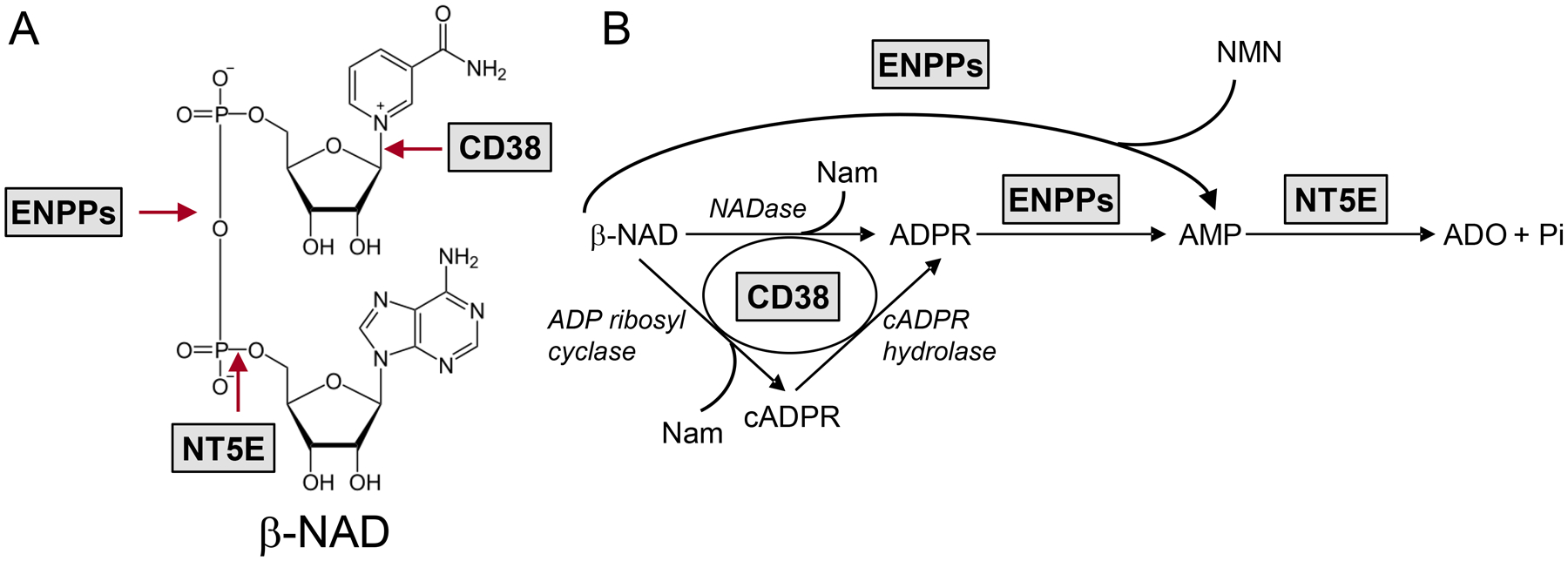
A. Chemical structure of β-NAD and sites of cleavage by ecto-enzymes. B. Extracellular biotransformation pathways for β-NAD demonstrating the sequence of product formation. CD38 serves as NAD glycohydrolase (NADase) (principal activity) as well as ADP ribosyl cyclase and cADPR hydrolase. ENPPs metabolize β-NAD and ADPR to AMP. NT5E degrades AMP to ADO. ENPPs, ecto-nucleotide pyrophosphatases; Nam, nicotinamide; NMN, nicotinamide mononucleotide.
