Figure 6.
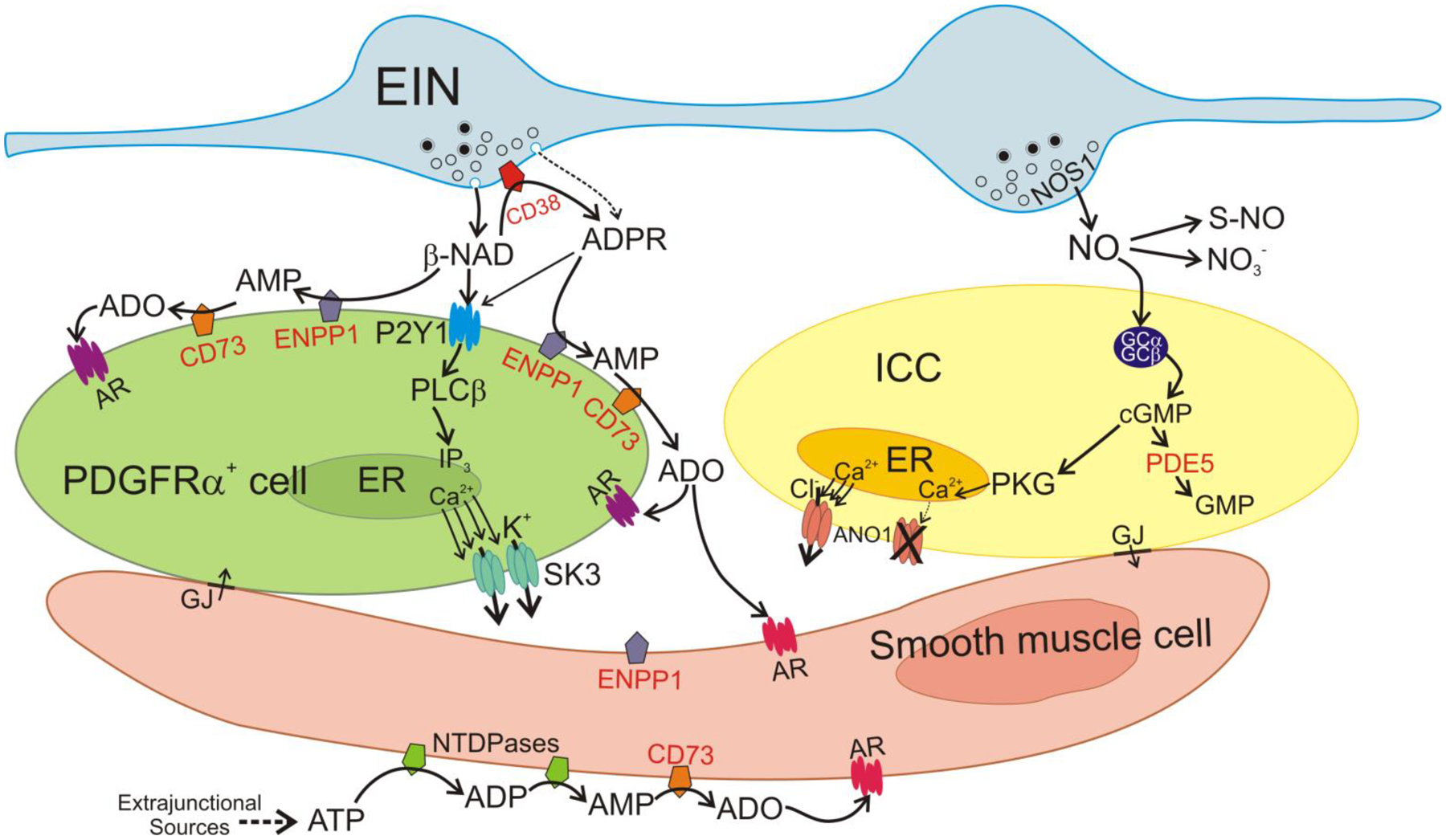
Mechanisms of non-peptide enteric inhibitory neurotransmitters and their metabolism. The SIP syncytium is composed of SMCs, ICC and PDGFRα+ cells, and it is innervated by enteric excitatory (not shown) and inhibitory motor neurons (EIN). Firing of EIN causes release of NO and β-NAD into the neuroeffector junction (NEJ). ADPR is also possibly released as a primary neurotransmitter (Durnin et al., 2012). The primary receptors mediating purinergic responses in the GI tract are P2Y1 receptors (Hwang et al., 2012, gallego et al., 2012, Gallego et al., 2006). P2Y1 receptors are dominantly expressed by PDGFRα+ cells (Peri et al., 2013). β-NAD binds to P2Y1 in the membrane of PDGFRα+ cells. P2Y1 receptors couple through Gq/11 to activate phospholipase Cβ (PLCβ) and generate IP3. IP3 binds to IP3 receptors in the ER membrane, increases the release of Ca2+ from stores and activates small-conductance K+ channels (SK3). SK3 channels generate outward currents that conduct to SMCs through gap junctions (GJ), producing a net inhibitory input of membrane hyperpolarization and reduced excitability and contractions of SMCs. β-NAD comes in contact with membrane-bound enzymes in the NEJ and is metabolized. CD38, likely present on EIN membranes, hydrolyses β-NAD to ADPR. ADPR also binds to P2Y1 and activates the same pathway activated by β-NAD. ADPR is also degraded to AMP by ENPP1expressed in PDGFRα+ cells. AMP is degraded to adenosine (ADO) by NT5E on membranes of PDGFRα+ cells and SMCs. ADO binds to adenosine receptors (AR) in PDGFRα+ cells and SMCs. Adora1 is the dominant AR expressed by PDGFRα+ cells (Ha et al., 2017). β-NAD can be directly degraded to AMP (bypassing ADPR formation) by ENPP1 on PDGFRα+ cells. Purine neurotransmitters and metabolites may also reach SMCs. SMCs express NT5E and hence high amounts of ADO can be formed. ADO can also bind to ARs on SMCs. The primary source for ATP in the colon tunica muscularis is extraneuronal (Durnin et al., 2013). ATP released from other sources that reaches SMCs is degraded by membrane-bound nucleotide tri- and diphosphatases (ENTDPases) to ADP and AMP. AMP is degraded to adenosine by NT5E in SMCs. NO released by EIN diffuses to ICC and SMCs (pathway in SMCs not shown) and binds to soluble guanylyl cyclase (sGS) that is made up of 2 subunits, GCα and GCβ. Binding of NO activates sGC and enhances production of cGMP which activates protein kinase G (PKG). Ca2+ is released in a stochastic manner spontaneously from the ER in ICC and activates Ca2+-activated Cl− channels (ANO1) (Drumm et al., 2019, Zhu et al., 2015). This generates an inward current that is conducted to SMCs via gap junctions and provides excitatory input to the SIP syncytium and increases contraction. PKG has multiple effects in ICC, but a major effect is to block Ca2+ release from the ER. This blocks activation of ANO1 and serves as a potent inhibitory signal in the SIP syncytium. NO is metabolized by oxidation to NO3− (intermediates not shown) or bound to activated thiol groups of proteins, forming S-nitrosothiols (S-NO). cGMP is metabolized by phosphodiesterases, mainly PDE5, which is strongly expressed in ICC and SMCs.
