Figure 1.
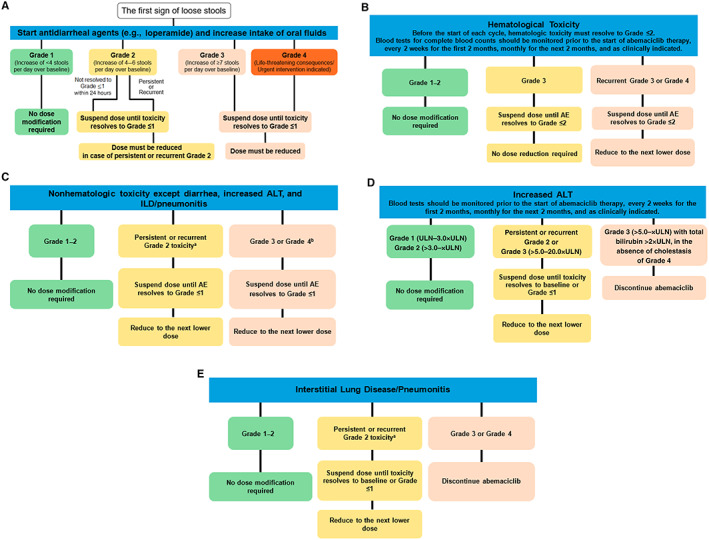
Recommendation for management of adverse events. (A), hematological toxicities (B), nonhematologic toxicities except diarrhea, increased ALT, and ILD/pneumonitis (C), increased ALT (D), and interstitial lung disease/pneumonitis (E) management. A dose reduction corresponds to a reduction of 50 mg of abemaciclib at a time. Discontinue abemaciclib for patients unable to tolerate 50 mg twice daily. For neutropenia evaluation, blood counts should be performed before starting abemaciclib treatment, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. If blood cell growth factors are administered, abemaciclib treatment must be suspended for at least 48 hours after the last administration of cell growth factors and until toxicity resolves to grade ≤2. Reduce the abemaciclib dose, unless already performed, for the toxicity that led to the use of growth factor. aGrade 2 toxicity that does not resolve with maximal supportive measures within 7 days to grade ≤1. bFor grade 4 increased aminotransferases, discontinue abemaciclib. Abbreviations: AE, adverse event; ALT, alanine aminotransferase; ILD, interstitial lung disease; ULN, upper limit of normal.
