Abstract
Background
Somatostatin analogs (SSAs) are the frontline antitumor therapy in advanced well‐differentiated gastroenteropancreatic neuroendocrine tumors (GEP‐NETs). A subset of patients demonstrate early disease progression on SSA therapy, yet the currently known predictors for treatment failure lack specificity to affect therapeutic decision. SSAs target tumor somatostatin receptors, the level of which can be quantitatively assessed with 68Ga‐DOTATATE positron emission tomography‐computed tomography (PET/CT). We investigated the ability of 68Ga‐DOTATATE PET/CT to predict response to SSA therapy.
Materials and Methods
The records of 108 consecutive patients with well‐differentiated grade 1–2 GEP‐NETs on SSA monotherapy who received 68Ga‐DOTATATE PET/CT scans were retrospectively reviewed to obtain baseline characteristics, 68Ga‐DOTATATE maximum standardized uptake value (SUVmax), and progression‐free survival (PFS) data. The optimal SUVmax cutoff for patient stratification was obtained with receiver operating characteristic curve analysis. PFS in the high versus low SUVmax groups was compared with Kaplan‐Meier survival analysis. The effects of baseline characteristics and SUVmax on PFS were examined with univariate and multivariate Cox regression.
Results
68Ga‐DOTATATE SUVmax predicted therapeutic failure with sensitivity and specificity of 39% and 98%, respectively. SUVmax of <18.35 was associated with shorter PFS, which was reproduced in the subgroup analysis of SSA‐naïve patients. Low SUVmax was the only predictor of early treatment failure (hazard ratio, 6.85) in multivariate analysis, as well as in the subgroup analysis of grade 2 GEP‐NETs.
Conclusion
Low SUVmax on 68Ga‐DOTATATE PET/CT independently predicts early failure on SSA monotherapy in patients with well‐differentiated grade 1–2 GEP‐NET. Patients with lack of expected benefit from SSA therapy can be readily identified using routine 68Ga‐DOTATATE PET/CT with very high specificity.
Implications for Practice
Based on 68Ga‐DOTATATE positron emission tomography‐computed tomography imaging, clinicians can better inform patients on the expected benefit of somatostatin analog therapy for gastroenteropancreatic neuroendocrine tumors, especially when access to the therapy is difficult, and offer proactive discussion on alternative management options.
Keywords: 68Ga‐DOTATATE, Positron emission tomography, Computed tomography, Octreotide, Lanreotide, Gastroenteropancreatic neuroendocrine tumor
Short abstract
Somatostatin analogs (SSAs) are the frontline antitumor therapy for patients with advanced well‐differentiated gastroenteropancreatic neuroendocrine tumors. This study investigated the ability of 68Ga‐DOTATATE PET/CT to predict response to SSA therapy.
Introduction
Gastroenteropancreatic neuroendocrine tumors (GEP‐NETs) represent approximately two thirds of neuroendocrine tumors (NETs) in adults, and 40%–50% of patients initially present with metastatic disease [1, 2]. Somatostatin analogs (SSAs), including octreotide and lanreotide, are used for treatment of advanced well‐differentiated GEP‐NETs and currently remain as the standard frontline antitumor therapy in this patient population despite the evolving role of peptide receptor radionuclide therapy (PRRT) and other therapeutics [3]. Although the response rate of SSA therapy remains low, treatment with these agents leads to disease stabilization in approximately two thirds of patients [4, 5]. There is, however, a subset of patients who demonstrate early disease progression on SSA therapy [6]. Previous studies for characterization of such patients identified several factors predictive of early treatment failure, including pancreatic primary tumor or fast tumor growth rate [7, 8], but none of the factors was specific enough to describe more than a tendency toward lack of response [6].
Because SSAs target tumor somatostatin receptors for their antitumor effect, it is common to perform baseline somatostatin receptor imaging such as 111In‐pentetreotide scintigraphy or 68Ga‐DOTATATE positron emission tomography‐computed tomography (PET/CT) before SSA therapy is initiated. The literature supporting this practice includes the placebo‐controlled PROMID and CLARINET trials, which demonstrated the efficacy of SSA therapy with patients with mostly 111In‐pentetreotide–positive GEP‐NET [4, 5], as well as a previous study that showed a higher response rate to SSAs in patients with GEP‐NET with positive 111In‐pentetreotide scan [9].
68Ga‐DOTATATE PET/CT scan, approved by the U.S. Food and Drug Administration in 2016, has revolutionized somatostatin receptor imaging with its superior spatial resolution and reduced scan time, as well as high sensitivity and specificity compared with the 111In‐pentetreotide imaging, with impact on patient management [10, 11, 12, 13]. Along with its analogs 68Ga‐DOTATOC and 68Ga‐DOTANOC, it has become the current gold standard of functional imaging for well‐differentiated NETs [14]. In contrast to 111In‐pentetreotide scintigraphy, 68Ga‐DOTATATE PET/CT provides quantitative information on tumor somatostatin receptor status in the form of standardized uptake values (SUVs), which correlate with somatostatin receptor expression levels on pathology [15].
SUVs on 68Ga‐DOTANOC PET/CT have overall prognostic value in the general GEP‐NET population [16, 17, 18]. More recently, higher SUVs on 68Ga‐DOTATOC and 68Ga‐DOTATATE PET/CT were found to predict response to PRRT [19, 20]. However, the role of quantitative radiotracer uptake measurements in predicting response to SSA therapy remains unclear. In the present study, we hypothesized that tumor uptake on 68Ga‐DOTATATE PET/CT measured by SUV can predict response to SSA therapy in well‐differentiated GEP‐NETs.
Materials and Methods
Study Population
Approval from the University of Pennsylvania Institutional Review Board was obtained prior to the study, and the requirement for informed consent was waived. The medical records of 528 consecutive patients who received 68Ga‐DOTATATE PET/CT scans at the University of Pennsylvania Health System from December 2016 to September 2018 were retrospectively reviewed. A final 108 patients with a pathologically confirmed diagnosis of well‐differentiated grade 1–2 GEP‐NETs treated with long‐acting depot SSA monotherapy (octreotide long‐acting release or lanreotide) were included in the study. At least 3 months of clinical follow‐up involving cross‐sectional imaging was required for enrollment. Patients managed with active surveillance, surgery, PRRT, liver‐directed therapy, or other systemic antitumor therapy for GEP‐NET were excluded from the study. Patients with active secondary malignancy, including NET from non‐gastroenteropancreatic origin, were excluded.
Review of 68Ga‐DOTATATE PET/CT
At least 3 weeks after the last SSA therapy, patients were given intravenous administration of 68Ga‐DOTATATE at a dose of 2 MBq/kg up to 200 MBq. No patients received short‐acting SSA around the time of their scan. Static positron emission tomography images were acquired from the skull base to midthigh 60 minutes after the radiotracer administration. An accompanying low‐dose non–contrast‐enhanced computed tomography was performed for attenuation correction and anatomic colocalization. Image processing and SUV measurements were performed retrospectively using MIM version 6 (MIM Software Inc., Cleveland, OH). SUV was corrected for total body weight. The maximum SUV (SUVmax) for each patient was defined as the highest SUV among primary and metastatic lesions.
Patient Assessments
The baseline clinical, pathologic, and laboratory characteristics of eligible patients were collected. Patients were then stratified based on gender, age, primary tumor site, tumor grade, Ki67 index, mitotic count, germline mutation status, tumor functionality, serum chromogranin A level, 24‐hour urine 5‐hydroxyindoleacetic acid (5‐HIAA) level, and exposure to SSA therapy prior to the 68Ga‐DOTATATE scan. Ki67 index cutoff of 5% was used as described previously [16, 17, 21]. Tumor functionality was determined based on presence of carcinoid syndrome for gastrointestinal tumors and overproduction of functionally active peptides for pancreatic tumors.
Progression‐free survival (PFS) was measured from the time of 68Ga‐DOTATATE scan for patients with prior SSA exposure and from the time of SSA therapy initiation for SSA‐naïve patients. PFS was evaluated radiologically according to RECIST version 1.1 [22].
Statistical Analysis
The patients were grouped according to their baseline characteristics, and the mean SUVmax among the groups was compared using one‐way analysis of variance (ANOVA). Log transformation of SUVmax was used for ANOVA to induce normality. The magnitude of difference in SUVmax was estimated by taking the antilog transformations of the ANOVA results and expressing them as percentages.
A receiver operating characteristic (ROC) curve was then constructed to determine the optimal SUVmax cutoff for predicting disease progression by 12 months. The area under the curve (AUC) was computed, and the optimal cutoff value was identified using the maximum likelihood estimation method [23]. Based on the cutoff, all 108 patients were stratified into high SUVmax and low SUVmax groups.
PFS in the high versus low SUVmax groups was compared with Kaplan‐Meier survival analysis using the Mantel‐Cox test. In addition, the effects of baseline characteristics and SUVmax on PFS were examined using univariate and multivariate Cox regression with 5% entry probability and 10% removal probability.
Using the same SUVmax cutoff, the PFS analysis was repeated on the subgroup of SSA‐naïve patients. In another subgroup analysis of patients with grade 2 tumors, the predictive value of SUVmax was compared with that of Ki67 index cutoff of 10% as proposed previously [5, 21, 24].
All statistical tests were two‐sided, and p values less than .05 were considered statistically significant. The statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA), except for Cox regression, which was performed with SPSS Premium 25 (SPSS Inc., Chicago, IL).
Results
Patient Characteristics
The baseline characteristics of the study population are summarized in Table 1. Eighty‐one patients (75%) were men, and twenty‐seven (25%) were women, with a median age of 60 (range, 27–85). All patients had metastatic disease, and there were more patients with gastrointestinal (75%) than pancreatic (25%) primary tumors. There were a similar number of patients with grade 1 (54%) versus grade 2 (46%) tumors. The median Ki67 index and mitotic count were 1% and 0 per 10 high‐power fields (HPFs) for grade 1 tumors and 5.6% and 1 per 10 HPFs for grade 2 tumors, respectively. Nine patients (8%) harbored NET‐related germline mutations, all of which involved the MEN1 gene. Forty‐nine patients (45%) had functioning tumors, six of which were pancreatic in origin (four gastrinomas, one glucagonoma, and one VIPoma). Serum chromogranin A and 24‐hour urine 5‐HIAA levels were elevated in 68% and 34% of patients, respectively. Thirty patients (28%) were SSA naïve at the time of the 68Ga‐DOTATATE scan.
Table 1.
SUVmax among different groups of 108 patients with gastroenteropancreatic neuroendocrine tumors on SSA monotherapy
| Baseline characteristics | Patients, n | Mean SUVmax ± SD | p value a | Effect size b (95% CI) |
|---|---|---|---|---|
| Gender | ||||
| Male | 81 | 57.9 ± 39.5 | .56 | |
| Female | 27 | 55.0 ± 40.5 | ||
| Age, years | ||||
| ≤50 | 20 | 63.8 ± 45.1 | .72 | |
| <50 and ≤60 | 38 | 56.0 ± 39.2 | ||
| <60 and ≤70 | 32 | 53.2 ± 39.2 | ||
| >70 | 18 | 53.7 ± 38.8 | ||
| Tumor site | ||||
| Gastrointestinal tract | 81 | 45.8 ± 28.4 | <.001 | 72.1% (57.1–88.4%) |
| Pancreas | 27 | 87.6 ± 52.0 | ||
| Tumor grade | ||||
| 1 | 53 | 57.7 ± 38.2 | .36 | |
| 2 | 46 | 56.1 ± 44.3 | ||
| Ki67 index | ||||
| ≤5% | 61 | 52.8 ± 35.6 | .97 | |
| >5% | 26 | 59.8 ± 49.2 | ||
| Mitotic count | ||||
| <2 per 10 HPFs | 55 | 55.5 ± 39.8 | .58 | |
| ≥2 per 10 HPFs | 22 | 59.9 ± 39.0 | ||
| Germline mutation | ||||
| Yes | 9 | 53.5 ± 36.9 | .89 | |
| No | 99 | 56.5 ± 40.3 | ||
| Functioning tumor | ||||
| Yes | 49 | 62.7 ± 31.7 | .16 | |
| No | 59 | 48.5 ± 44.9 | ||
| Chromogranin A | ||||
| Normal (≤95 ng/mL) | 34 | 63.0 ± 46.0 | .87 | |
| Elevated (>95 ng/mL) | 71 | 53.4 ± 37.0 | ||
| Urine 5‐HIAA | ||||
| Normal (≤15 mg/day) | 39 | 53.6 ± 38.2 | .24 | |
| Elevated (>15 mg/day) | 20 | 40.8 ± 20.8 | ||
| SSA naïve | ||||
| Yes | 30 | 53.0 ± 36.1 | .69 | |
| No | 78 | 57.5 ± 41.4 |
Comparisons among groups were made using one‐way analysis of variance (ANOVA) on log‐transformed SUVmax data.
Effect size was estimated by taking antilog transformations of the ANOVA results.
Abbreviations: 5‐HIAA, 5‐hydroxyinidoleacetic acid; CI, confidence interval; HPF, high‐power field; SSA, somatostatin analog; SUVmax, maximum standardized uptake value.
Effect of Baseline Characteristics on SUVmax
The means and SDs of SUVmax among different groups of patients are shown in Table 1. SUVmax was 72.1% higher (95% confidence interval [CI], 57.1%–88.4%; p < .001) in pancreatic compared with gastrointestinal NETs. However, SUVmax did not vary with any other baseline characteristics, including gender (p = .56), age (p = .72), tumor grade (p = .36), Ki67 index (p = .97), mitotic count (p = .58), germline mutation status (p = .89), tumor functionality (p = .16), tumor marker levels (p = .24–0.87), and prior SSA therapy (p = .69).
Follow‐Up
From the time of 68Ga‐DOTATATE scan, the median duration of clinical follow‐up was 16 months (range, 3–24 months). Probability of freedom from progressive disease at 6, 12, and 18 months, reported as mean ± SEM, was 90.9 ± 2.9%, 80.0 ± 4.3%, and 60.8 ± 6.3%, respectively (Fig. 1A). There was no difference in PFS between gastrointestinal and pancreatic primary tumors (p = .50; Fig. 1B). Overall, 30 patients (27.5%) demonstrated disease progression by the last follow‐up, with median PFS of 8.5 months among them.
Figure 1.
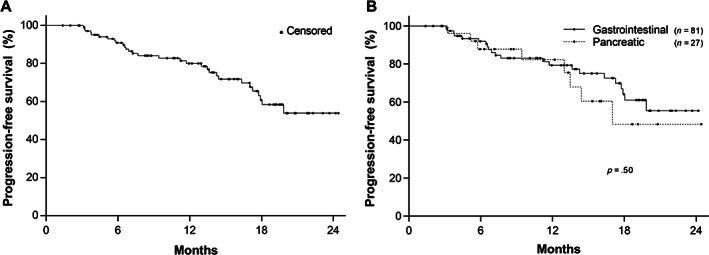
Kaplan‐Meier plot of progression‐free survival in 108 patients with grade 1–2 gastroenteropancreatic neuroendocrine tumors on somatostatin analog monotherapy. (A): Probability of progression‐free survival at 6, 12, and 18 months was 90.9% ± 2.9%, 80.0% ± 4.3%, and 60.8% ± 6.3%, respectively (mean ± SEM). (B): There was no significant difference in progression‐free survival between the primary tumor sites (p = .50).
ROC Curve Analysis
The ROC curve of 68Ga‐DOTATATE SUVmax for predicting disease progression is shown in Figure 2 (AUC = 0.66, p = .043 for AUC ≠ 0.5). The optimal SUVmax cutoff determined was 18.35, yielding the maximum likelihood ratio of 21.4. The sensitivity and specificity for disease progression within 12 months based on SUVmax <18.35 were 38.9% (95% CI, 22.7%–58.0%) and 98.2% (95% CI, 92.3%–99.8%), respectively.
Figure 2.
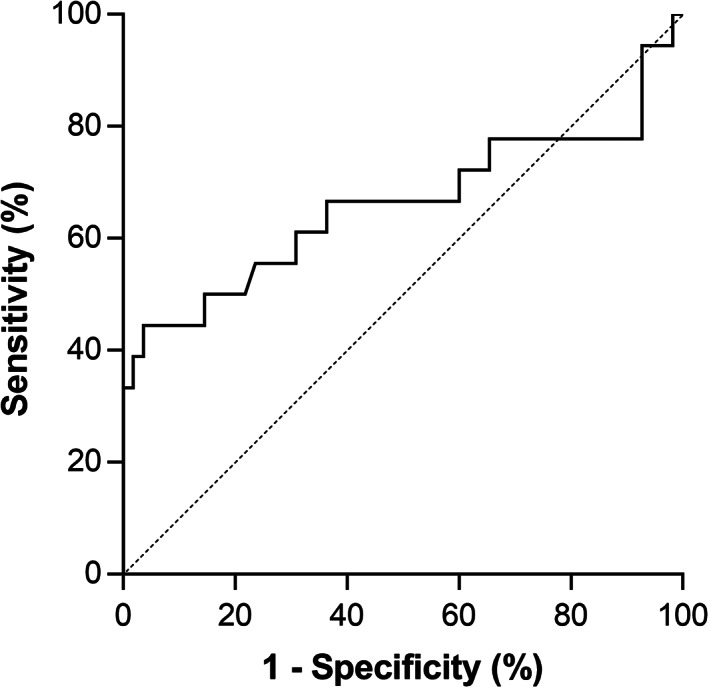
Receiver operating characteristic curve of 68Ga‐DOTATATE maximum standardized uptake value (SUVmax) for predicting disease progression within 12 months. Sensitivity and specificity obtained using the best cutoff value (SUVmax <18.35, likelihood ratio = 21.4) were 38.9% and 98.2%, respectively. Area under the curve (AUC) = 0.66 with p = .043 for AUC ≠ 0.5.
Progression‐Free Survival Analysis
The Kaplan‐Meier survival analysis revealed significantly shorter PFS in the low SUVmax group compared with the high SUVmax group (p < .0001; Fig. 3A) in the overall study population, with median PFS of 6.6 versus >24 months, respectively (median follow‐up, 6.4 vs. 13.3 months). This trend was reproduced in the subgroup analysis of 30 SSA‐naïve patients (p = .019; Fig. 3B), with median PFS of 5.7 versus >19 months, respectively (median follow‐up, 4.4 vs. 10.7 months).
Figure 3.

Kaplan‐Meier plots of progression‐free survival in patients with grade 1–2 gastroenteropancreatic neuroendocrine tumors on somatostatin analog (SSA) monotherapy based on 68Ga‐DOTATATE maximum SUV (SUVmax) cutoff of 18.35. Progression‐free survival was significantly shorter in the low SUVmax group, both among all 108 patients (p < .0001 on Mantel‐Cox test) (A) and among 30 SSA‐naïve patients (p = .019) (B). Abbreviation: SUV, standardized uptake value.
On univariate Cox regression analysis, higher tumor grade (p = .002), Ki67 index (p = .010), and mitotic count (p = .007), as well as low SUVmax (p < .001) and no prior SSA therapy (p = .034), were identified as predictors of early failure on SSA monotherapy (Table 2). However, only SUVmax <18.35 remained statistically significant on multivariate analysis with hazard ratio (HR) of 6.85 (95% CI, 2.10–22.34; p = .001). When the multivariate analysis was repeated with a different 68Ga‐DOTATATE SUVmax cutoff of 21.6, which was previously demonstrated to predict response to PRRT [19], similar results were obtained with an HR of 5.31 (95% CI, 1.63–17.33, p = .006).
Table 2.
Risk/protective factors for disease progression in patients with gastroenteropancreatic neuroendocrine tumors on SSA monotherapy
| Parameter | Univariate | Multivariate a | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Gender (male vs. female) | 1.35 (0.66–2.77) | .412 | ||
| Age (increasing) | 1.07 (0.75–1.53) | .695 | ||
| Site of primary tumor (GI vs. pancreas) | 0.76 (0.34–1.70) | .499 | ||
| Grade (1 vs. 2) | 0.27 (0.12–0.61) | .002 | ||
| Ki67 (≤5% vs. >5%) | 0.37 (0.17–0.81) | .010 | ||
| Mitotic count (<2 vs. ≥2 per 10 HPFs) | 1.17 (1.04–1.31) | .007 | NA | |
| Germline mutation (yes vs. no) | 1.63 (0.49–5.47) | .428 | ||
| Functioning tumor (yes vs. no) | 0.64 (0.31–1.32) | .220 | ||
| Chromogranin A (normal vs. elevated) | 0.91 (0.42–1.97) | .805 | ||
| Urine 5‐HIAA (normal vs. elevated) | 1.32 (0.43–4.06) | .627 | NA | |
| SSA naïve (yes vs. no) | 2.33 (1.06–5.12) | .034 | ||
| SUVmax (<18.35 vs. ≥18.35) | 4.15 (1.88–9.15) | <.001 | 6.85 (2.10–22.34) | .001 |
Only statistically significant (p < .05) results are reported for multivariate analysis.
Abbreviations: 5‐HIAA, 5‐hydroxyinidoleacetic acid; CI, confidence interval; GI, gastrointestinal; HPF, high‐power field; HR, hazard ratio; NA, not applicable (not included in multivariate analysis because of inconsistent reporting); SSA, somatostatin analog; SUVmax, maximum standardized uptake value.
In the subgroup of 46 patients with grade 2 tumors, Kaplan‐Meier survival analysis also revealed shorter PFS with SUVmax <18.35 (p = .006; Fig. 4A), with median PFS of 6.7 versus 17.3 months in low versus high SUVmax groups, respectively (median follow‐up, 6.4 vs. 11.2 months). In contrast, there was no statistically significant difference in PFS when the patients with grade 2 tumors were stratified based on Ki67 index cutoff of 10% (p = .38; Fig. 3B). Similarly, univariate Cox regression showed that SSA therapy failure was associated with SUVmax <18.35 (HR, 3.65; 95% CI, 1.35–9.87; p = .011), but not with Ki67 index cutoff of 10% (p = .380). Therefore, Ki67 index had no further value in predicting response to SSA monotherapy among patients with grade 2 GEP‐NET, whereas SUVmax remained informative.
Figure 4.

Kaplan‐Meier plots of progression‐free survival among 46 patients with grade 2 gastroenteropancreatic neuroendocrine tumors on somatostatin analog monotherapy. (A): Maximum SUV (SUVmax) <18.35 predicted early disease progression (p = .006 on Mantel‐Cox test) with hazard ratio of 3.65 (95% confidence interval: 1.35–9.87, p = .011) on univariate Cox regression. (B): Stratification according to Ki67 index cutoff of 10% (excluding one patient with missing value) did not reveal statistically significant differences in progression‐free survival (p = .38). Abbreviation: SUV, standardized uptake value.
Discussion
In the present study, we evaluated the utility of SUV measurements on 68Ga‐DOTATATE PET/CT for predicting progression‐free survival on SSA therapy in patients with well‐differentiated GEP‐NET. SUVs in pancreatic NETs were higher than those of gastrointestinal NETs, as previously found with both 68Ga‐DOTANOC and 68Ga‐DOTATATE [16, 24]. The finding is in accordance with the higher expression levels of somatostatin receptor subtype 2 (SSTR2) in pancreatic NETs [25], as 68Ga‐DOTATATE is a specific ligand for SSTR2 and its degree of uptake is strongly correlated with tumor SSTR2 content [26, 27]. The lack of variation in SUVmax with the other baseline characteristics suggests that 68Ga‐DOTATATE PET/CT is an independent source of clinical information in the patient population.
The high specificity (98.2%) for treatment failure predicted by low SUVmax illustrates that a sufficient level of SSTR2 expression is a requirement for successful SSA therapy even among SSTR2‐positive tumors, as previously found histopathologically [28]. On the other hand, the relatively lower sensitivity (38.9%) of the SUVmax cutoff can be explained by two factors. First, the SUVmax only takes into account the tumor with the highest SSTR2 level in a given patient, and heterogeneity of SSTR2 expression may lead to local SSA therapy failure even in the setting of high SUVmax. Second, resistance to SSA therapy may result from multiple biological mechanisms downstream of receptor‐ligand binding, which would decouple tumor 68Ga‐DOTATATE uptake and efficacy of SSA therapy [29]. Therefore, the biological basis of SSA therapy explains the observation that meeting the threshold SUVmax is a necessary, but not sufficient, condition for response to SSA therapy.
We found a statistically significant difference in PFS based on the SUVmax cutoff, and a closer look at the PFS suggests potential futility of SSA therapy in the low SUVmax group. The PFS of 5.7 months we found in the low SUVmax group is comparable to or shorter than the placebo arm PFS of 6 months and 18 months in the PROMID and CLARINET studies, respectively [4, 5]. On the other hand, the PFS of >24 months in the high SUVmax group is longer than or comparable to the treatment arm PFS of the two studies (14.3 months and >24 months). Hence, the short PFS in the low SUVmax group we found may represent complete lack of response to SSA therapy rather than early treatment failure.
SSA therapy is generally well tolerated, with relatively minor side effects mostly involving the gastrointestinal system, such as diarrhea and flatulence [5, 30]. However, serious adverse events related to SSA therapy and subsequent study withdrawal were noted in both PROMID and CLARINET studies [4, 5]. A perhaps larger drawback of SSA therapy is the associated cost. A 2017 U.S.‐based analysis estimated the total cost of SSA therapy, including management of adverse effects, at $74,566 for octreotide and $84,856 for lanreotide during the first year of treatment [31]. Such prohibitive cost makes access to SSA therapy difficult. Furthermore, because it takes about 6 months to detect progression on a futile therapy in this relatively indolent disease, typically at least half of the first year's cost will be incurred even without any clear benefit. Therefore, in the setting of likely lack of benefit, the time and resources required for initiation, maintenance, and monitoring of SSA therapy may be directed toward proactive discussion of alternative therapy options. Therefore, identifying the group of patients with minimal to no expected benefit from SSA therapy has critical value in the management of patients with GEP‐NET.
The results of the multivariate regression analysis suggest that the best estimator for identification of such a group of patients is SUVmax on 68Ga‐DOTATATE PET/CT. Although the pathologic variables (tumor grade, Ki67 index, and mitotic count) were also able to predict response to SSA on univariate analysis, their statistical significance was lost on multivariate analysis. Similarly, SUVmax on 68Ga‐DOTANOC PET/CT was previously identified as an independent overall prognostic marker superior to the pathologic variables [16, 17]. Interestingly, lack of prior SSA therapy also predicted early failure on SSA therapy on univariate analysis. This finding is likely attributed to selection bias, because the patients already on SSA therapy represent those without prior evidence of treatment failure. The unique role of 68Ga‐DOTATATE PET/CT in predicting response to SSA therapy was also demonstrated in comparison with Ki67 index in the subgroup analysis of patients with grade 2 GEP‐NET.
The most notable clinical implication of the present study is prediction of treatment failure with very high specificity, which has not been possible with previously known predictive factors [6]. With increasing integration of 68Ga‐DOTATATE PET/CT into the initial workup of patients with well‐differentiated GEP‐NET, SUVmax for a given patient can be easily obtained without demand for additional resources. The subgroup analysis of SSA‐naïve patients demonstrates the utility of using 68Ga‐DOTATATE PET/CT as a screening measure prior to initiating SSA therapy, as is routinely done for PRRT currently. We also found that 68Ga‐DOTATATE SUVmax can be used to guide therapy for all patients including those currently on SSA, in accordance with a previous study that demonstrated no significant change in mean and maximum 68Ga‐DOTATATE SUVs with SSA therapy in both primary and metastatic lesions [32].
A major limitation of using SUVmax cutoff to predict response to SSA therapy is variability in SUV measurements. Previously, somatostatin receptor–targeted PET/CT in patients with GEP‐NET showed temporal variability in tumor SUVmax measurements within 25% [33, 34]. Across imaging sites, 18F‐fluorodeoxyglucose–based studies revealed practical variability in SUVmax measurements of at least 15%–20% [35]. Therefore, the SUVmax cutoff used for possibly withholding SSA therapy should include a margin of safety to account for this variability. In addition, the treatment risk‐benefit discussion with patients based on 68Ga‐DOTATATE uptake should involve disclosure of the variability in SUVmax measurements.
Despite the variability, the SUVmax cutoff of 18.35 we found is similar to those of previous studies for prediction of overall prognosis and response to PRRT. Campana et al. and Sharma et al. each found the best 68Ga‐DOTANOC SUVmax cutoff of 17.9–19.3 and 14.5, respectively, to be an overall prognostic marker in the general GEP‐NET population [16, 17]. Previously proposed cutoff for predicting response to PRRT was 16.4 for 68Ga‐DOTANOC and 21.6 for 68Ga‐DOTATATE [19, 20]. Although Koch et al. reported a relatively higher SUVmax cutoff of 29.35 for predicting response to octreotide therapy [36], the difference is mainly attributed to the ROC curve analysis method. In our study, we prioritized specificity over sensitivity in order to minimize withholding of potentially beneficial SSA therapy, whereas Koch et al. aimed to maximize the overall accuracy of response prediction. By minimizing the distance to the upper left corner of the ROC curve, we can also obtain a similar SUVmax cutoff of 33.10 with sensitivity and specificity of 61% and 69%, respectively. Overall, we believe clinical implementation of SUVmax cutoff would be feasible, especially considering routine use and clinical utility of Ki67 index despite its substantial variability at intralesional, interlesional, temporal, and interlaboratory levels [37, 38, 39, 40].
SUVmax measurements are also significantly affected by tumor volume. Partial volume effects in small lesions results in falsely low SUVmax measurements and therefore compromise the specificity of predicting SSA therapy failure. However, we were able to maintain very high specificity in our study, likely because of the limited role of small lesions in radiologic response assessment and sufficient number of large lesions in the patients to capture the overall tumor behavior with SUVmax, especially because we considered only the single highest SUV value in each patient [41]. Also, uptake in normal organs can be falsely low in patients with large tumor burden because of sink effect [42]. Nevertheless, global tumor SUVmax in a given patient increases with the total 68Ga‐DOTATATE avid tumor volume [43], explained by the wider range of SUVs offered by the larger number of lesions. The patients with larger tumor volume and SUVmax progress faster naturally [43], but the longer PFS we observed in our study suggests efficacy of SSA therapy that trumps natural disease progression in this group of patients. Because tumor volume remains as a potential source of bias, combined use of tumor volume and SUVmax cutoffs in a larger number of patients may lead to more accurate response prediction to SSA therapy.
Although the present study was conducted retrospectively, stratification of patients into low versus high SUVmax groups cannot be random, and the patients were enrolled consecutively in the order they were scanned. Therefore, a prospective study design is not required to validate the differences we found between the two groups, as evidenced by the similarity of our study design to previous prospective studies [16, 17]. On the other hand, the main confounder of our study is the possibility that shorter PFS in the low SUVmax group merely depicts aggressive tumor phenotype rather than lack of response to SSA therapy. Demonstrating lack of therapeutic benefit in the low SUVmax group will require validation in a placebo‐controlled prospective study involving only the patients with low SUVmax. The generalizability of our results to the general GEP‐NET population also remains to be investigated.
Conclusion
A subset of patients with grade 1–2 GEP‐NETs demonstrate early disease progression on SSA monotherapy. Low SUVmax on 68Ga‐DOTATATE PET/CT independently identifies such patients with very high specificity, whereas conventional clinicopathologic parameters such as Ki67 index provide only limited information on response to SSA. We present quantitative use of somatostatin receptor–targeted PET/CT for predicting response to SSA therapy, which can aid clinicians in the risk‐benefit analysis regarding initiation and maintenance of SSA therapy.
Author Contributions
Conception/design: Hwan Lee
Provision of study material or patients: Hwan Lee
Collection and/or assembly of data: Hwan Lee
Data analysis and interpretation: Hwan Lee, Jennifer R. Eads, Daniel A. Pryma
Manuscript writing: Hwan Lee, Jennifer R. Eads, Daniel A. Pryma
Final approval of manuscript: Hwan Lee, Jennifer R. Eads, Daniel A. Pryma
Disclosures
Jennifer R. Eads: Novartis (SAB), Lexicon (C/A), Pfizer (H), Bristol‐Myers Squibb (E); Daniel A. Pryma: Siemens AG, 511 Pharma, Progenics Pharmaceuticals Inc. (RF), Progenics Pharmaceuticals Inc., 511 Pharma, Siemens AG, Actinium Pharmaceuticals Inc., Bayer. (C/A). Hwan Lee indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Nicole Brighi, Francesco Panzuto, Roberta Modica et al. Biliary Stone Disease in Patients with Neuroendocrine Tumors Treated with Somatostatin Analogs: A Multicenter Study. The Oncologist 2020;25:259–265.
Implications for Practice: The results of this study confirm an increased rate of gallstones development and related complications in patients with neuroendocrine tumors (NETs) treated with somatostatin analogs (SSAs). NETs of the gastrointestinal (GI) tract and related surgery are independent risk factors for biliary stone disease development. Therefore, all patients with primary GI‐NET or undergoing abdominal surgery should be considered for prophylactic cholecystectomy. Data on other subgroups are not exhaustive, and management also evaluating additional clinical features (life expectancy, surgical and anesthesiological risks) should be considered. Prophylactic treatment with ursodeoxycholic acid does not seem to be a protective factor for SSA‐related biliary stone disease.
References
- 1. Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pavel M, O'Toole D, Costa F et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016;103:172–185. [DOI] [PubMed] [Google Scholar]
- 3. Uri I, Grozinsky‐Glasberg S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP‐NETs). Clin Diabetes Endocrinol 2018;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rinke A, Muller HH, Schade‐Brittinger C et al. Placebo‐controlled, double‐blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol 2009;27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 5. Caplin ME, Pavel M, Cwikla JB et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 6. Sideris L, Dube P, Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. The Oncologist 2012;17:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panzuto F, Di Fonzo M, Iannicelli E et al. Long‐term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well‐differentiated entero‐pancreatic endocrine carcinoma. Ann Oncol 2006;17:461–466. [DOI] [PubMed] [Google Scholar]
- 8. Aparicio T, Ducreux M, Baudin E et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer 2001;37:1014–1019. [DOI] [PubMed] [Google Scholar]
- 9. Janson ET, Westlin JE, Eriksson B et al. [111In‐DTPA‐D‐Phe1]octreotide scintigraphy in patients with carcinoid tumours: The predictive value for somatostatin analogue treatment. Eur J Endocrinol 1994;131:577–581. [DOI] [PubMed] [Google Scholar]
- 10. Sadowski SM, Neychev V, Millo C et al. Prospective study of 68Ga‐DOTATATE positron emission tomography/computed tomography for detecting gastro‐entero‐pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol 2016;34:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schreiter NF, Brenner W, Nogami M et al. Cost comparison of 111In‐DTPA‐octreotide scintigraphy and 68Ga‐DOTATOC PET/CT for staging enteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2012;39:72–82. [DOI] [PubMed] [Google Scholar]
- 12. Hofman MS, Kong G, Neels OC et al. High management impact of Ga‐68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol 2012;56:40–47. [DOI] [PubMed] [Google Scholar]
- 13. Mojtahedi A, Thamake S, Tworowska I et al. The value of (68)Ga‐DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: A review of literature. Am J Nucl Med Mol Imaging 2014;4:426–434. [PMC free article] [PubMed] [Google Scholar]
- 14. Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015;35:500–516. [DOI] [PubMed] [Google Scholar]
- 15. Kaemmerer D, Peter L, Lupp A et al. Molecular imaging with (6)(8)Ga‐SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2011;38:1659–1668. [DOI] [PubMed] [Google Scholar]
- 16. Campana D, Ambrosini V, Pezzilli R et al. Standardized uptake values of (68)Ga‐DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J Nucl Med 2010;51:353–359. [DOI] [PubMed] [Google Scholar]
- 17. Sharma P, Naswa N, Kc SS et al. Comparison of the prognostic values of 68Ga‐DOTANOC PET/CT and 18F‐FDG PET/CT in patients with well‐differentiated neuroendocrine tumor. Eur J Nucl Med Mol Imaging 2014;41:2194–2202. [DOI] [PubMed] [Google Scholar]
- 18. Ambrosini V, Campana D, Polverari G et al. Prognostic value of 68Ga‐DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med 2015;56:1843–1848. [DOI] [PubMed] [Google Scholar]
- 19. Sharma R, Wang WM, Evans J et al. 68Ga‐DOTATATE PET/CT to predict response to peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumours (NETs). J Clin Oncol 2017;35(suppl 15):4093a. [DOI] [PubMed] [Google Scholar]
- 20. Kratochwil C, Stefanova M, Mavriopoulou E et al. SUV of [68Ga]DOTATOC‐PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol 2015;17:313–318. [DOI] [PubMed] [Google Scholar]
- 21. Jamali M, Chetty R. Predicting prognosis in gastroentero‐pancreatic neuroendocrine tumors: An overview and the value of Ki‐67 immunostaining. Endocr Pathol 2008;19:282–288. [DOI] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 23. Metz CE, Herman BA, Shen JH. Maximum likelihood estimation of receiver operating characteristic (ROC) curves from continuously‐distributed data. Stat Med 1998;17:1033–1053. [DOI] [PubMed] [Google Scholar]
- 24. Zhang P, Yu J, Li J et al. Clinical and prognostic value of PET/CT imaging with combination of (68)Ga‐DOTATATE and (18)F‐FDG in gastroenteropancreatic neuroendocrine neoplasms. Contrast Media Mol Imaging 2018;2018:2340389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Toole D, Saveanu A, Couvelard A et al. The analysis of quantitative expression of somatostatin and dopamine receptors in gastro‐entero‐pancreatic tumours opens new therapeutic strategies. Eur J Endocrinol 2006;155:849–857. [DOI] [PubMed] [Google Scholar]
- 26. Heidari P, Wehrenberg‐Klee E, Habibollahi P et al. Free somatostatin receptor fraction predicts the antiproliferative effect of octreotide in a neuroendocrine tumor model: Implications for dose optimization. Cancer Res 2013;73:6865–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reubi JC, Schar JC, Waser B et al. Affinity profiles for human somatostatin receptor subtypes SST1‐SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000;27:273–282. [DOI] [PubMed] [Google Scholar]
- 28. Qian ZR, Li T, Ter‐Minassian M et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas 2016;45:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 2003;24:28–47. [DOI] [PubMed] [Google Scholar]
- 30. Oberg K, Kvols L, Caplin M et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004;15:966–973. [DOI] [PubMed] [Google Scholar]
- 31. Ayyagari R, Neary M, Li S et al. Comparing the cost of treatment with octreotide long‐acting release versus lanreotide in patients with metastatic gastrointestinal neuroendocrine tumors. Am Health Drug Benefits 2017;10:408–415. [PMC free article] [PubMed] [Google Scholar]
- 32. Ayati N, Lee ST, Zakavi R et al. Long‐acting somatostatin analog therapy differentially alters (68)Ga‐DOTATATE uptake in normal tissues compared with primary tumors and metastatic lesions. J Nucl Med 2018;59:223–227. [DOI] [PubMed] [Google Scholar]
- 33. Coura‐Filho GB, Hoff A, Duarte PS et al. 68Ga‐DOTATATE PET: Temporal variation of maximum standardized uptake value in normal tissues and neuroendocrine tumours. Nucl Med Commun 2019;40:920–926. [DOI] [PubMed] [Google Scholar]
- 34. Menda Y, Ponto LL, Schultz MK et al. Repeatability of gallium‐68 DOTATOC positron emission tomographic imaging in neuroendocrine tumors. Pancreas 2013;42:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kinahan PE, Fletcher JW. Positron emission tomography‐computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR 2010;31:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koch W, Auernhammer CJ, Geisler J et al. Treatment with octreotide in patients with well‐differentiated neuroendocrine tumors of the ileum: Prognostic stratification with Ga‐68‐DOTA‐TATE positron emission tomography. Mol Imaging 2014;13:1–10. [PubMed] [Google Scholar]
- 37. Grillo F, Albertelli M, Brisigotti MP et al. Grade increases in gastroenteropancreatic neuroendocrine tumor metastases compared to the primary tumor. Neuroendocrinology 2016;103:452–459. [DOI] [PubMed] [Google Scholar]
- 38. Singh S, Hallet J, Rowsell C et al. Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. Eur J Surg Oncol 2014;40:1517–1522. [DOI] [PubMed] [Google Scholar]
- 39. Panzuto F, Cicchese N, Partelli S et al. Impact of Ki67 re‐assessment at time of disease progression in patients with pancreatic neuroendocrine neoplasms. PLoS One 2017;12:e0179445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Focke CM, Burger H, van Diest PJ et al. Interlaboratory variability of Ki67 staining in breast cancer. Eur J Cancer 2017;84:219–227. [DOI] [PubMed] [Google Scholar]
- 41. Chalian H, Töre HG, Horowitz JM et al. Radiologic assessment of response to therapy: Comparison of RECIST versions 1.1 and 1.0. Radiographics 2011;31:2093–2105. [DOI] [PubMed] [Google Scholar]
- 42. Beauregard JM, Hofman MS, Kong G et al. The tumour sink effect on the biodistribution of 68Ga‐DOTA‐octreotate: Implications for peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2012;39:50–56. [DOI] [PubMed] [Google Scholar]
- 43. Tirosh A, Papadakis GZ, Millo C et al. Prognostic utility of total (68)Ga‐DOTATATE‐avid tumor volume in patients with neuroendocrine tumors. Gastroenterology 2018;154:998–1008.e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]


