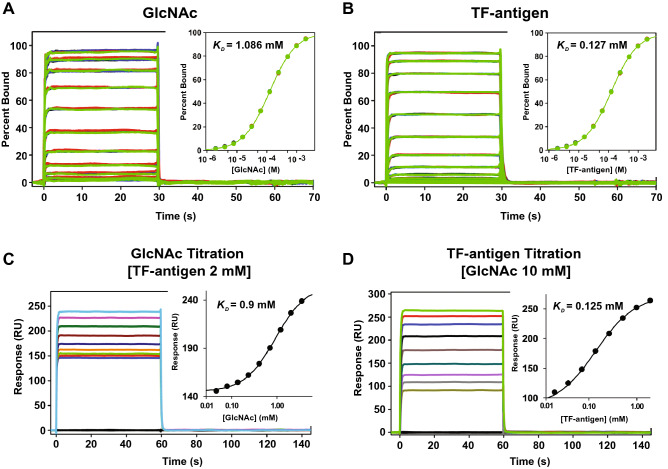
Figure 5.
Ligand binding analysis of wild-type rBGL using SPR. (A) GlcNAc and (B) TF-antigen (Galβ1,3-GalNAc) were tested for binding to immobilized rBGL using a twofold concentration series up to 10 mM and 2 mM, respectively. Shown are sensorgrams captured for 8 replicate binding experiments (overlayed colored lines). Two competition binding experiments were run with the rBGL surface. (C) TF-antigen concentration was fixed at 2 mM while GlcNAc was tested in a twofold concentration series up to 5 mM. (D) GlcNAc concentration was fixed at 10 mM while TF-antigen was tested in a twofold concentration series up to 2 mM. For all experiments, equilibrium dissociation binding constants were determined by fitting data at equilibrium to a 1:1 interaction model using the software Scrubber 2. Fit curves are shown in insets.