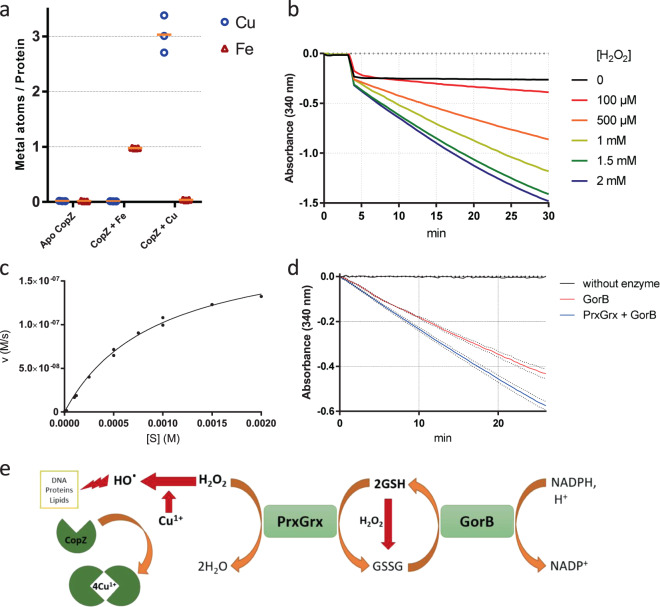
Fig. 5. Activities of the proteins coded by the bp1727-1728-1729 (copZ-prxgrx-gorB) operon.
a The metal/protein ratios of recombinant CopZ were determined by ICP-AES. The apo form of CopZ was incubated or not with iron (gray) or copper (black), and both ions were measured. Three independent samples were processed. b Oxidation of NADPH by recombinant GorB over time. H2O2 was added at the indicated concentrations to generate the glutathione disulfide (GSSG) substrate of GorB, before adding the enzyme. The reaction was followed by the decrease of absorbency at 340 nm. c Plot of the reaction rate as a function of substrate concentration. A kcat /Km value of 323,000 ± 46,000 M−1s−1 for GSSG was estimated based on Michaelis–Menten kinetics. One or two measurements were performed at each substrate concentration. d Effect of recombinant PrxGrx on the rate of oxidation of NADPH by GorB. The red and blue curves show reaction rates when GorB was present alone or when both enzymes were present, respectively. H2O2 was added last as a substrate of the first reaction. However, as H2O2 also generates GSSG, which initiates the second reaction, PrxGrx activity was detected by the increased rate of the reaction when both enzymes are present, and no enzymatic constants could be determined. (e) Schematic representation of the functions of the three proteins. By chelating Cu1+, CopZ prevents the ion from generating hydroxyl radicals through the Fenton reaction. H2O2 is reduced to H2O through the activity of PrxGrx, at the expense of reduced glutathione (GSH). The product of that reaction, glutathione disulfide, is reduced through the activity of GorB at the expense of NADPH.