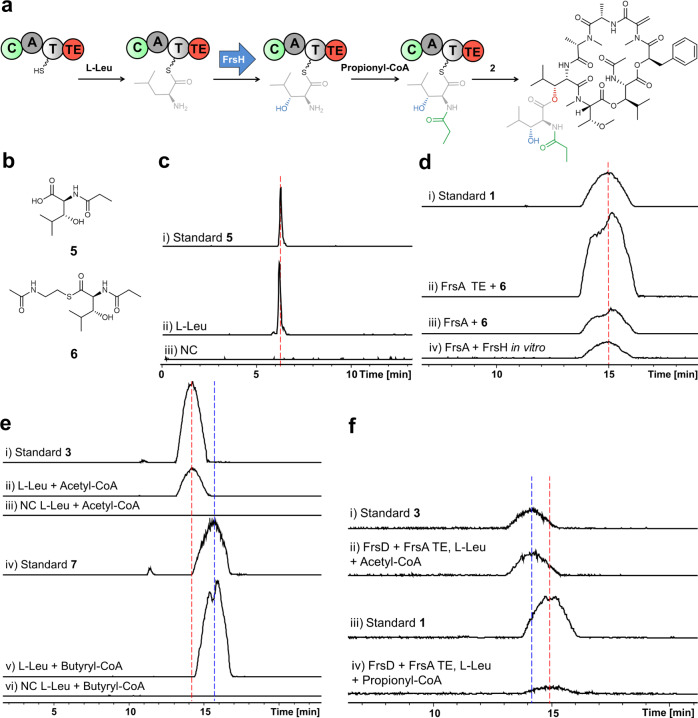
Fig. 3. In vitro assays with FrsA/B, FrsH, and FrsATE.
a Reaction scheme of N-Pp-Hle formation and intermolecular transesterification onto 2 to yield 1 Biosynthetic modifications are color-coded according to the respective catalyzing domain. b Structures of synthesized N-Pp-Hle (5) and its SNAC (6). c In vitro production of 5 (m/z: 202.108). (i) Synthetic 5 (1 µg/ml). (ii) Enzymatic assay with purified FrsACAT/FrsB, FrsH, incubated with l-Leu and propionyl-CoA, hydrolyzed with KOH. (iii) Negative control (NC) with heat-inactivated protein. d In vitro production of 1 (m/z: 1002.540). (i) 1 (10 µg/ml). (ii) Purified FrsATE incubated with 6 and 2. (iii) Purified FrsA/B incubated with 6 and 2. (iv) Purified FrsA/B, FrsH incubated with propionyl-CoA, l-Leu and 2. e In vitro production of FR congeners 3 (m/z: 988.530) and 7 (m/z: 1016.550). (i) 3 (10 µg/ml). (ii) Purified FrsA/B, FrsH incubated with l-Leu, acetyl-CoA and 2. (iii) Negative control. (iv) 7 (10 µg/ml). (v) Purified FrsA/B, FrsH incubated with l-Leu, butyryl-CoA and 2. (vi) Negative control. f In vitro FrsA/D combinations: (i) 3 (1 µg/ml). (ii) Production of 3 by FrsATE and FrsD, incubated with acetyl-CoA, l-Leu and 2. (iii) 1 (1 µg/ml). (iv) Production of 1 by FrsATE and FrsD, incubated with propionyl-CoA, l-Leu and 2.