Abstract
Most solid-state materials are composed of p-block anions, only in recent years the introduction of hydride anions (1s2) in oxides (e.g., SrVO2H, BaTi(O,H)3) has allowed the discovery of various interesting properties. Here we exploit the large polarizability of hydride anions (H–) together with chalcogenide (Ch2–) anions to construct a family of antiperovskites with soft anionic sublattices. The M3HCh antiperovskites (M = Li, Na) adopt the ideal cubic structure except orthorhombic Na3HS, despite the large variation in sizes of M and Ch. This unconventional robustness of cubic phase mainly originates from the large size-flexibility of the H– anion. Theoretical and experimental studies reveal low migration barriers for Li+/Na+ transport and high ionic conductivity, possibly promoted by a soft phonon mode associated with the rotational motion of HM6 octahedra in their cubic forms. Aliovalent substitution to create vacancies has further enhanced ionic conductivities of this series of antiperovskites, resulting in Na2.9H(Se0.9I0.1) achieving a high conductivity of ~1 × 10–4 S/cm (100 °C).
Subject terms: Batteries, Batteries, Batteries
Oxide-based lithium/sodium-rich antiperovskites are regarded as promising solid electrolytes. Here, authors report a series of antiperovskites with a soft lattice containing hydride (H–) and chalcogenide (S2–, Se2–, Te2–) anions, enabling the fast Li+ /Na+ transport assisted by rotational phonon modes.
Introduction
Perovskites with a formula of ABX3 (A, B: cations, X: anions) are ubiquitous and central to electronics, photonics, and energy technologies1,2. Their electronically inverted analogs, antiperovskites, are playing an increasingly major role in solid-state chemistry and physics owing to their various intriguing properties, such as giant magnetoresistance in Mn3GaC3, superconductivity in Ni3MgC4, negative thermal expansion in Mn3BA (B = Zn, Cu etc.; A = N, C)5,6, efficient water splitting electrocatalytic activity in Ni3FeN7, superionic conductivity in Ag3SI8 and large capacity in (Li2Fe)OCh (Ch = S, Se, Te) as lithium battery cathodes9.
In particular, lithium-rich and sodium-rich antiperovskites (LiRAPs and NaRAPs), for example, M3OCl, M3OBr (M = Li, Na), and Na3OBH4, have recently attracted a great deal of attention as they exhibit a high lithium (or sodium) ionic conductivity and are thus regarded as promising solid-state electrolytes enabling high-energy-density lithium metal batteries10–13. Similar to the F– superionic conductivity in NaMgF3 perovskite14, the antiperovskite M3OCl allows Li+/Na+ superionic conductivity benefited from the M-rich content (60 at% in Li3OCl)10,13. Another advantage of antiperovskite is its extraordinary chemical diversity. Similar to perovskite, a variety of combinations of elements can be accommodated, while maintaining the simple structural topology, thereby offering an ideal situation to easily and fully characterize fast ionic transport15,16. A very recent study on Li3OCl indicated that the presence of hydroxide (OH–), providing the composition of Li3–xO1–x(OH)xCl, or ultimately Li2(OH)Cl17.
In the search of new fast ion-conducting solid-state materials18–27, a great deal of effort has been made to understand ionic transport in Li-ion conductors, where two features of the anion-host matrix have been highlighted18–20. The first feature proposed by Ceder et al. is that the anion arrangement of body-centered cubic (bcc) provides the lowest migration barrier for Li-ion diffusion, rather than a close-packed (fcc or hcp) anion framework27. Antiperovskites host the bcc framework composed of A- and B-site anions10,13,16. The second feature is that polarizable anions can critically soften and flatten the cationic transport landscape, leading to lower activation energy and higher ionic conductivity23,25,28–31. In fact, thiophosphate ionic conductors, such as Li10GeP2S12 with the highest Li-ion conductivity, are believed to benefit from a soft and polarizable anion lattice32.
In this study, we present the synthesis of a series of LiRAPs and NaRAPs, M3HCh (M = Li, Na; Ch = S, Se, Te) with both anionic sites occupied by soft and polarizable anions of H– and Ch2– (Fig. 1)33,34. To our knowledge, LiRAPs and NaRAPs have been obtained only with oxide/hydroxide anions at the B site, which considerably limits the scope of structures and properties. Experimental and theoretical investigations have revealed that the occupation of highly polarizable and size-flexible H– anions at the B site in M3HCh introduces several interesting features, including the stability and robustness of the ideal cubic structure and softening of phonon mode associated with HM6 octahedral rotation, which could be advantageous for realizing high Li+/Na+ conductivity. The energy migration barriers based on vacancy and interstitial dumbbell mechanisms are found to be lower than those of oxide-based LiRAPs and NaRAPs. Li+/Na+ vacancy creation via iodine (I–) doping (for Ch2–) is a potential strategy to increase the ionic conductivity of this series of antiperovskites, where I-doped cubic Na3HSe with formula Na2.9H(Se0.9I0.1) delivers a high total Na+ conductivity exceeding 1 × 10–4 S/cm at 100 °C with a low bulk activation energy of ~0.18 eV in agreement with the calculated one (~0.16 eV).
Fig. 1. Crystal structure of hydride-based M3HCh antiperovskite (M = Li, Na).
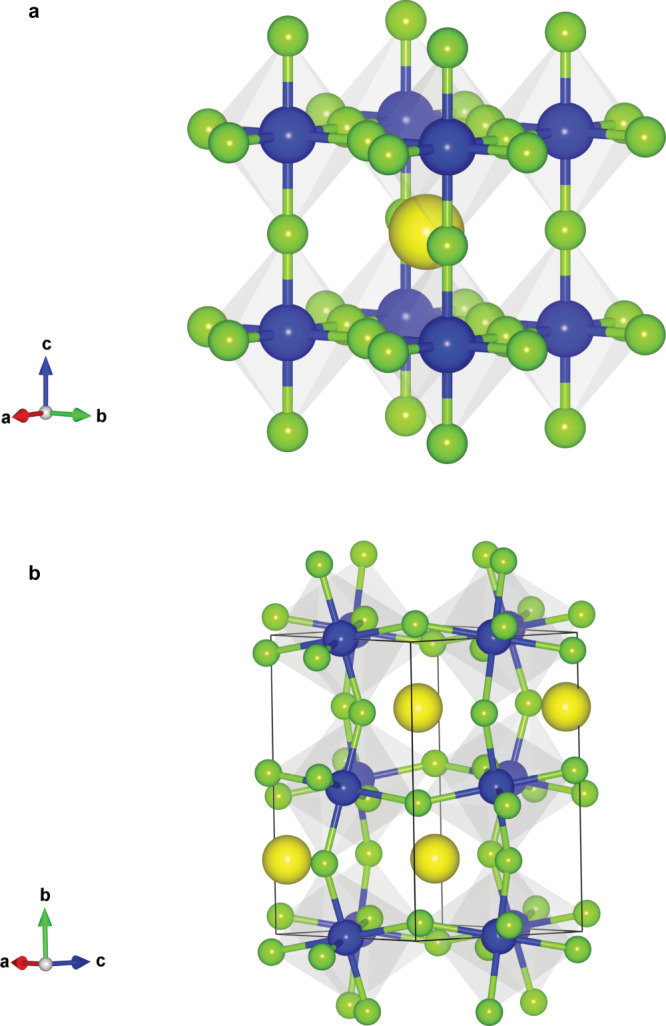
a Crystal structure of the cubic antiperovskite (space group Pm–3 m). In this study, we obtained Li3HCh (Ch = S, Se, Te) and Na3HCh (Ch = Se, Te), where H– anion (blue; B site) is bonded with Li+/Na+ cations (green) forming HLi6/HNa6 octahedron, while chalcogenide anion (yellow; A site) sits in cuboctahedral coordination site. As opposed to conventional perovskites, the cubic structure is robust in a wide range of compositions. b The orthorhombic anti-GdFeO3 type (space group Pnma) Na3HS with HNa6 octahedral tilting.
Results
Structure determination
The high-resolution synchrotron X-ray diffraction (SXRD) pattern of the sample prepared using LiH and Li2S at 5 GPa and 700 °C (Fig. 2a) shows the formation of a highly crystalline compound, along with impurity phases that could be identified as LiH, Li2S, and BN (insulating high-pressure sleeve). The diffraction profile of the main phase was indexed in a cubic system with the lattice parameter of a = 3.85189(6) Å (see Supplementary Fig. 1), which is comparable to those of reported antiperovskite oxyhalides Li3OCl and Li3OBr (a = 3.900 Å and 3.989 Å, respectively) with the space group Pm–3m10,12.
Fig. 2. Structural determination of Li3HS.
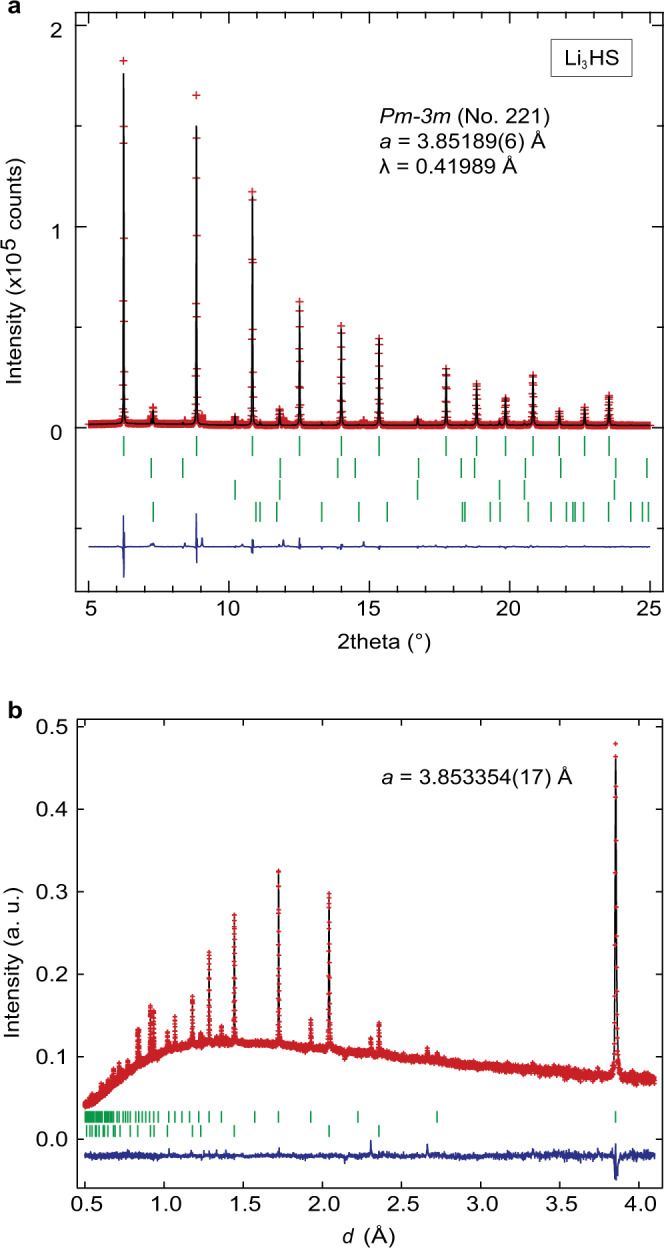
Rietveld refinement of a SXRD and b ND. The red crosses, black solid line, blue solid line, and green dashes denote, respectively, the observed, calculated, difference intensities, and calculated Bragg reflections (from top to bottom: Li3HS, Li2S, LiH, BN in a; Li3HS, LiH in b).
Given the known antiperovskites, e.g., Ag3SI8, Li3OCl10, with a smaller anion at the octahedral B site and a larger one at the cuboctahedral A site, we performed Rietveld refinement assuming S2– at the 1b (1/2, 1/2, 1/2) Wyckoff position and H– at the 1a (0, 0, 0) site and Li+ at the 3d (1/2, 0, 0) site within the space group of Pm-3m, corresponding to a stoichiometric Li3HS formula. The Rietveld refinement converged successfully with values of RBragg = 4.28% and Rf = 2.87% (see Fig. 2a and Supplementary Table 1). Swapping the anionic octahedral B site and cubocathedral A site to give a Li3SH led to poor refinement values of RBragg = 22.6% and Rf = 11.2% (Supplementary Fig. 2).
Subsequently, time-of-flight powder neutron diffraction (ND) data were analyzed to better characterize lighter elements of H and Li. The presence of hydrogen in the sample can be readily seen from Fig. 2b exhibiting a high background intensity. The neutron refinement assuming the Li3HS structure yielded Rwp = 1.35% and RBragg = 6.28% thus confirming this structural model. Details of the refinement are listed in Supplementary Table 1. It is found that the Li+, H–, and S2– ions fully occupy their respective crystallographic sites, confirming the stoichiometric composition. We also examined the possibility of antisite disorder between H– and S2–; the Rietveld refinement allowing intersite anion exchange (Supplementary Table 1) in the stoichiometric composition led to the full occupancy (g) of H– at the B site (gB(H) = 1.015(5)) with Rwp = 1.37% and RBragg = 6.64%, which implies that the anions are perfectly ordered. Note that Pd3H0.89In also has hydrogen at the octahedral site35, but this material may not be classified as hydride, given the metallic nature of this material and the nearly identical electronegativity between H (2.2) and Pd (2.2)33. Attempts to synthesize Li3HS compound at a lower pressure (1 GPa/3 GPa; 700 °C) or under ambient pressure (700 °C for 12 h) using LiH and Li2S was unsuccessful (Supplementary Fig. 3), indicating the high-pressure metastability of this antiperovskite.
Materials variety
To our knowledge, Li3HS is the first LiRAP with hydride anions at the B site. Next, we attempted to extend the antiperovskite family by including sodium as well as other chalcogenides under high-pressure and high-temperature reactions. The XRD patterns of Li3HSe and Li3HTe (Supplementary Fig. 4) are similar to Li3HS, with Bragg reflections moving toward lower angles, as expected from increasing ionic radii of chalcogenide anions. The obtained cell parameters of the cubic unit cell are 3.9744(5) Å for Li3HSe and 4.2221(3) Å for Li3HTe. Regarding the sodium system, the SXRD profiles of Na3HSe (Supplementary Fig. 5) and Na3HTe (Supplementary Fig. 6) are compatible with the cubic symmetry (Pm–3m) with a = 4.55901(7) Å and a = 4.76349(2) Å, respectively. The larger cell parameters result from the difference in the cationic size (Na+: 1.02 Å vs. Li+: 0.76 Å)36. Rietveld refinements of Na3HSe and Na3HTe validated the cubic antiperovskite structure, with detailed structural information in Supplementary Tables 2 and 3.
To gain microscopic information on the crystal structure, we conducted 23Na nuclear magnetic resonance (NMR) with/without magic-angle spinning (MAS) for Na3HSe. The spectra in Supplementary Fig. 7 were fitted as a second-order quadrupolar line shape of the central transition with a common set of CQ = 1.61 MHz and η = 0.04, where CQ, and η denote a quadrupole coupling constant and an asymmetry parameter, respectively. The 23Na MAS NMR spectrum shows a single sharp peak at 24.2 ppm, which indicates that all sodium atoms are in the same environment, in accordance with the refinement result that no significant chemical disorder occurs in Na3HSe (Supplementary Fig. 5 and Supplementary Table 2). This isotropic chemical shift (i.e., 24.2 ppm) is fairly consistent with that derived from DFT calculation (20.4 ppm with CQ = 1.95 MHz and ŋ DFT = 0).
In the case of Na3HS, the XRD pattern (Supplementary Fig. 4) is clearly different and indexed by an orthorhombic unit cell (a = 6.76037(9) Å, b = 8.89761(10) Å, and c = 6.28659(8) Å), which is related to the pristine cubic cell by √2ap × 2 bp × √2cp. This supercell and the extinct reflections suggest that Na3HS adopts an anti-GdFeO3 structure (Pnma space group, Fig. 1b), as previously reported for M3OA (M = Sr, Eu, Ba, and A = Si, Ge)37. SXRD (Supplementary Fig. 8) and ND (Supplementary Fig. 9) data were refined assuming the anti-GdFeO3 structure, yielding reasonable parameters, as listed in Supplementary Tables 4 and 5.
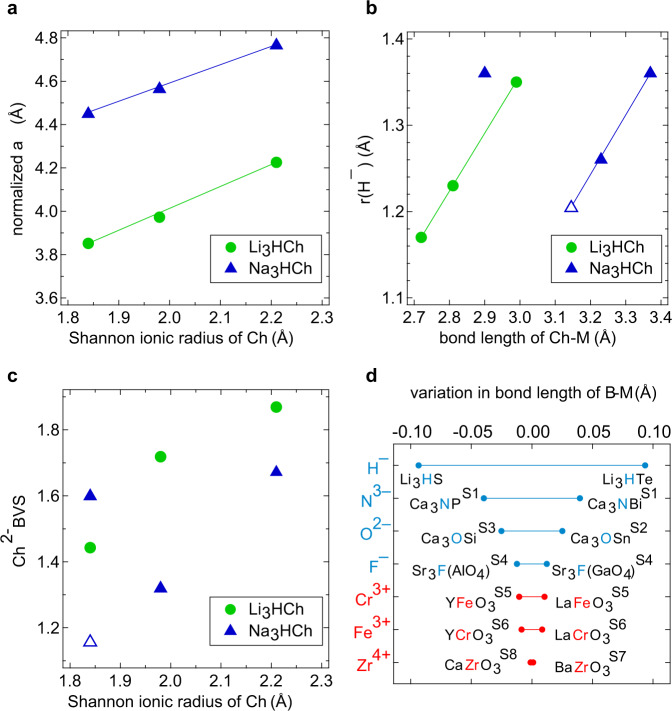
As shown in Fig. 3a, the normalized (cubic) lattice parameters of the LiRAP and NaRAP series both show linear dependence as a function of the chalcogen ionic radii, with approximately the same slope. As it is commonly done for normal perovskite structures, the tolerance factor (t) was estimated for our system, assuming the hydride ionic radius of 1.40 Å36,38. We found that the cubic structures are observed over a wide range 0.85 < t < 0.97 (Supplementary Table 6), in sharp contrast to perovskite oxides where slight deviations from unity readily lead to structural distortions. It is also noticed that oxide-based antiperovskites M3OA (M = Ca, Sr, Ba, Eu; A = Si, Ge, Sn, Pb) exhibit octahedral tilting when t < 0.9737, implying that our hydride-based system is quite anomalous. The t value of the orthorhombic Na3HS is 0.84, slightly smaller than that of cubic Li3HS (t = 0.85).
Fig. 3. Structural features of M3HCh antiperovskites (M = Li, Na).
a Normalized (cubic) lattice parameters of M3HCh. For orthorhombic o-Na3HS, the cubic lattice parameter is an average of normalized lattice parameters. Lines are for the eye guidance. b The hydride anion (H–) size obtained by subtracting M+ radius from H––M+ bond length. Open triangles indicate the ionic radius of H– in hypothetical cubic c-Na3HS, which is calculated by using the average normalized cubic lattice parameter of the orthorhombic o-Na3HS. c BVS values for Ch2–. Open triangles indicate the S2– BVS for hypothetical cubic c-Na3HS. d Variation in bond length of B–M in BM6 (B = H–, N3–, O2–, F–) octahedra for M3BA antiperovskites and B–O in BO6 (B = Fe3+, Cr3+, Zr4+) octahedra for ABO3 perovskites. The mentioned studies refer to Supplementary References (S1–S8).
Phase stability
Earlier studies on oxyhydrides have shown that the hydride anion (H–) adapts itself to the different local environments, resulting in the hydride size flexibility; this unique feature brings about a number of novel properties such as high-pressure-induced coordination reversal in LaHO33,39–42. This means that our assumption of the fixed hydride size (1.40 Å) for calculating the tolerance factor was not adequate. Hence, we estimated the ionic radius of hydride anions, r(H–) by subtracting the ionic radii of alkali metals, r(M+) from the H–M bond lengths (a/2 in the case of the cubic system). Figure 3b shows that the hydride substantially changes its size, with r(H–) ranging from 1.17 Å to 1.36 Å. For the Li3HCh series, r(H–) expands nearly linearly with the Ch–M bond length down the chalcogen group, in line with the increasing volume of HLi6 octahedron and ChLi12 cuboctahedron (Supplementary Fig. 10). A similar linear dependence can be recognized as long as the cubic phase of Na3HCh (including the hypothetical cubic c-Na3HS) is considered. Interestingly, the hydride size in the real orthorhombic o-Na3HS deviates from this linear relationship; r(H–) in o-Na3HS greatly increases to the value closer to cubic Na3HTe. The particularly soft anion (H–) with the flexible size is counterintuitive to the traditional hard-sphere model with fixed ionic radii when describing the bonding nature of ionic compounds.
To better understand the swollen H– in orthorhombic o-Na3HS, we calculated bond valence sum (BVS) values of chalcogenide using the tabulated parameters43. Note that the size flexibility of hydride anion does not permit a reliable estimate of BVS for the hydride anion itself40. As shown in Fig. 3c, the BVS value for S2– in hypothetical c-Na3HS is –1.16, which is unusually low, indicating that S2– is greatly underbonded in the cuboctahedral site. Here, the flexible hydride comes into play. In o-Na3HS, the HNa6 octahedron (due to swollen H–) is greatly expanded (as displayed in Supplementary Fig. 10), which in turn reduces the SNa12 volume and allows S2– to gain an acceptable BVS value of –1.60. For the Li3HCh series, Li3HS has the lowest Ch-BVS value of –1.44, which is higher than that for Na3HSe (–1.32).
Since we have estimated r(H–) for each compound (Fig. 3b), we can redefine the tolerance factor (t’) (see Supplementary Table 6). It is found that cubic hydride antiperovskites have large t’ from 0.93 to 0.99, while orthorhombic Na3HS has t’ = 0.85. The redefined t’ for Li3HCh is closer to unity and has a narrower range. For example, Li3HS with a minimum t of 0.85 changes to t’ = 0.95 when the observed r(H–) is applied. Therein lies the extraordinary size flexibility of hydride, that is, the marked variation in bond length of H–Li in HLi6 octahedra, from a maximum of 2.11 Å for Li3HTe to a minimum of 1.93 Å for Li3HS, as shown in Fig. 3d. The obtained bond-length difference of 0.09 Å for hydride antiperovskites is much larger than those of other antiperovskites with B = N (0.04 Å), O (0.03 Å), and F (0.01 Å). However, from a broader perspective, antiperovskites generally have larger octahedral size variations than conventional oxide perovskites, developing the potential to tailor structures toward acquiring new functions.
Until now, we had a sharp picture of the large size variation of hydride anion. The chalcogenide anion, however, is also polarizable. To critically evaluate the role of anions in stabilizing the cubic structure, we estimated the radius of each ion using Bader population analysis, which partitions the first-principles-calculated charge density grid into the Bader region of each ion (see Methods in Supplementary Information)44. The resulting Bader radius of Li+ only increases from 0.94 to 0.97 Å when the A-site chalcogenide anion increase from S2– to Te2–, whereas the Bader radius of H– ion expands from 1.38 to 1.48 Å (Supplementary Table 7). The large size variation of H– is also evidenced in Na3HCh. Interestingly, the change in Bader radius of Ch2– ions is comparable to that of H– ions; the Bader radii of Se2– and H– are 2.06 and 1.42 Å, respectively, in Li3HSe and increase to 2.24 and 1.52 Å in Na3HSe. However, when applying the external pressure (5 GPa) to M3HCh, the Bader radius of H– decreases more substantially than that of Ch2– (Supplementary Fig. 11). The hydride ion is more sensitive to the applied pressure than chalcogenide. Given that most of M3HCh compounds are currently synthesized by high pressure, we argue that the flexible hydride is more critical for stabilizing the cubic symmetry.
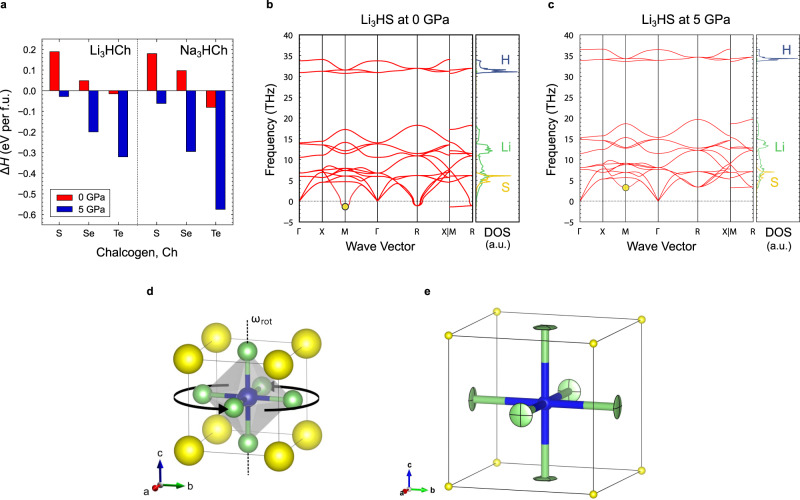
To investigate the thermodynamic stability of this series of hydride antiperovskites, we evaluated the formation enthalpy ΔH of M3HCh (M = Li, Na) in the reaction (MH + M2Ch → M3HCh) under 0 and 5 GPa based on first-principles calculations, where ΔH is defined as ΔH = H(M3HCh) − {H(M2Ch) + H(MH)}. Calculations showed that Li3HS, Na3HS, and Na3HSe are thermodynamically unstable at 0 GPa (ΔH > 0), but are stabilized under high pressure (Fig. 4a). On the other hand, Li3HTe and Na3HTe with large t’ values (0.99 and 0.96, respectively) are stable even at ambient pressure. Hence, the proof-of-concept trial to synthesize Na3HTe was conducted by heating a mixed pellet of NaH and Na2Te at 400 °C overnight in a vacuum-sealed Pyrex tube. The resulting SXRD pattern (Supplementary Fig. 12) yielded a cubic phase with a = 4.76717(5) Å, which is identical with the sample obtained under high pressure (a = 4.76349(2) Å) and antisite anion disorder exists in neither case (Supplementary Table 8). In addition, the Gibbs free energy difference (ΔG) showed that Li3HSe is stable at ambient pressure (ΔG = −0.01 eV/f.u.), despite the slightly positive value of ΔH (Fig. 4a); our preliminary synthesis under simple ambient conditions has failed, but there remains room for adjusting parameters, such as partial H2 gas pressure.
Fig. 4. Formation enthalpy and phonon calculations.
a Formation enthalpy ΔH in the reaction MH + M2Ch → M3HCh (M = Li, Na) under 0 and 5 GPa. b Phonon band structures and partial phonon density of states (DOS) of Li, H, and S atoms in Li3HS under 0 GPa and c 5 GPa. The negative number of the vertical axis represents imaginary frequency. d HM6 octahedral rotation associated with the phonon mode denoted by yellow circles in phonon band dispersion, where ωrot is the frequency of the HM6 rotational mode at M point. e Displacement ellipsoids of Li atoms depicted at 50% probability level obtained from Rietveld refinement of ND in Li3HS.
Electron and phonon calculations
The electronic band structures at 0 GPa (Supplementary Fig. 13) obtained using first-principles calculations show that all the compounds have relatively large bandgaps: 4.2 eV for Li3HS, 3.5 eV for Li3HSe, 2.9 eV for Li3HTe, 2.9 eV for Na3HS, 2.8 eV for Na3HSe and 2.7 eV for Na3HTe. Although GGA-PBE exchange-correlation functional generally underestimates bandgaps45, our compounds are expected to have electronically good insulating properties to meet the requirements as a solid electrolyte.
Phonon band structures of the hydride antiperovskites at 0 and 5 GPa were calculated using lattice dynamics within harmonic approximation (see Fig. 4b, c for Li3HS and Supplementary Fig. 14 for the others), including the hypothetical cubic c-Na3HS. All the synthesized cubic compounds are found to be dynamically stable at 5 GPa. In contrast, the c-Na3HS under 0 and 5 GPa exhibits imaginary phonon modes at the M and R points, corresponding to in-phase and out-of-phase rotations of the HM6 octahedral rotation, respectively46, which as a result confirms the experimentally observed orthorhombic structure o-Na3HS with the a+b–b– tilting in Glazer notation. Li3HSe, Li3HTe, and Na3HTe are dynamically stable even at 0 GPa without any imaginary phonon frequencies. For Li3HS and Na3HSe (dynamically stable at 5 GPa), imaginary phonon modes (lattice instabilities) appear at M and R points under 0 GPa, which is inconsistent with experimental observations of the cubic phase after quenching from 5 GPa; this inconsistency might be resolved by including anharmonic effects of phonons at finite temperatures, which is the case of cubic SrTiO347.
The calculated phonon dispersions of M3HCh (M = Li, Na) and atom-projected phonon DOS are quite unique; high frequencies associated with optical phonons are ascribed to local vibrations of the lighter H– ion, whereas vibrations by heavier M+ and Ch2– ions are responsible for low phonon frequencies, along with relatively flat dispersions. Furthermore, the phonon band center of M+ ions decreases as the Ch2– ion becomes larger (e.g., 9.6 to 9.2 to 8.4 THz for Li3HCh), suggesting a tunability of lattice softness for mobile M+ ions. Given the concept of “the softer the lattice, the better”18,20,21,23,25,31 and the relatively large calculated bandgap, M3HCh antiperovskites should be a promising candidate as Li/Na solid-state electrolytes. In the next section, we will explore ionic transport properties in this series of hydride-based antiperovskites from a theoretical and experimental point of view.
Ionic conductivity
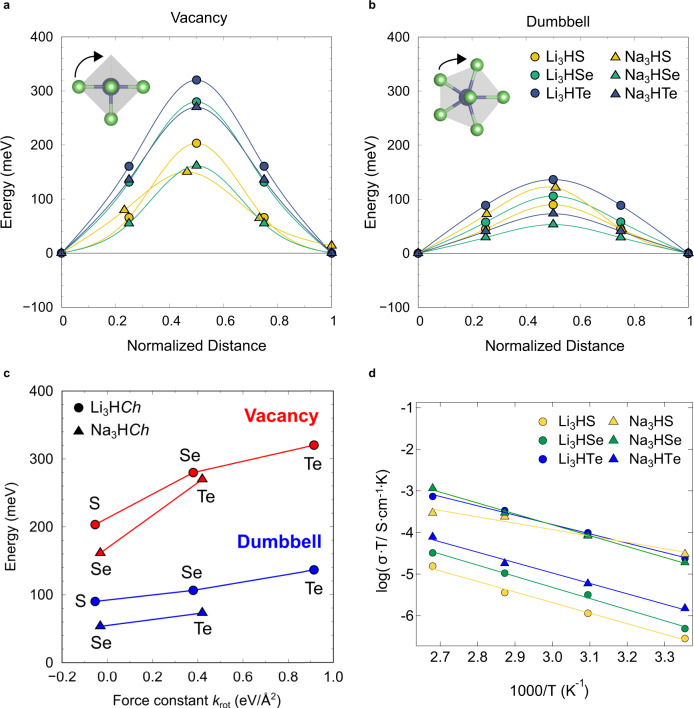
Nudged elastic band (NEB) calculations were used to estimate the energy barrier for cationic transport. Previous studies on Li3OCl proposed two transport mechanisms, i.e., Li hopping via vacancy mechanism and Li interstitial dumbbell hopping along edges of LiO6 octahedra10,12. We calculated migration barriers for Li/Na transport in M3HCh based on these two mechanisms, and the results are displayed in Fig. 5a for the vacancy mechanism and Fig. 5b for the dumbbell mechanism. The energy barriers via vacancy mechanism range from 0.15 eV (o-Na3HS, Supplementary Fig. 15) to 0.32 eV (Li3HTe), while the dumbbell mechanism has a much smaller energy barrier from 0.05 eV (Na3HSe) to 0.14 eV (Li3HTe). The calculated energy barriers via both of mechanisms for M3HCh are notably low, and the difference of more than half between two mechanisms is also observed in previously reported antiperovskites (e.g., in Li3OCl, 0.31 eV and 0.15 eV for vacancy and dumbbell mechanisms, respectively)12. It should also be noted that the calculated migration barrier (3.49 eV) for hydride (H–) hopping via vacancy in Li3HS (Supplementary Fig. 16) is much higher than that of Li+ (0.20 eV), indicating that H– anions hardly migrate in our antiperovskite. From the results of potentiostatic measurement of the symmetric cell Li3PS4/Li2.9H(S0.9I0.1)/Li3PS4, the steady-state current suggesting the sole motion of Li+ in iodine-doped Li3HS is observed in Supplementary Fig. 17.
Fig. 5. Ionic conductivities of M3HCh antiperovskites (M = Li, Na, and Ch = S, Se, Te).
Low-barrier migration pathways for a vacancy and b interstitial dumbbell migration in M3HCh. Top-left insets show M+ migration when viewing HM6 octahedron from the top to down. For orthorhombic Na3HS, there are many migration pathways, one of which is shown as a representative example (see Supplementary Fig. 15 for details). c Migration barriers for cation transport via the vacancy and interstitial dumbbell mechanism in M3HCh as a function of the force constant krot with respect to the HM6 rotational motion. d Arrhenius plots of the total conductivity values for undoped M3HCh cold-pressed samples in the temperature range from 25 to 100 °C.
To our surprise, the energy barrier is largest for Ch = Te, followed by Se and S. This seems counterintuitive since the cell volume increases (corresponding to the widening of the channels for Li/Na transport) as the ionic radius of chalcogenide ion increase from S to Se and then Te. At the same time, the phonon band center of Li/Na becomes lower (enhancing the softness of lattice), which implies that other factors such as local lattice dynamics are playing a role in ion diffusion. A systematic investigation of phonon dispersions in M3HCh revealed that the rotational motion of the HM6 octahedron reflecting on the phonon mode at M point (Fig. 4d) is essential to understand the unusual Ch dependence. This rotational motion corresponds well to the migration direction of M+ ions to adjacent sites and displacement ellipsoids (anisotropic atomic displacement parameters) of Li/Na (Fig. 4e) obtained from the Rietveld refinement conform well to the rotational mode. The frequency of this phonon mode, ωrot, decreases with decreasing the Ch2– size (1.4i, 3.7 and 5.7 THz for Li3HS, Li3HSe and Li3HTe and 0.6i and 2.1 THz for Na3HSe and Na3HTe), demonstrating the softening of this specific rotational phonon. For both Li/Na migration mechanism, ωrot2·mM (where mM is the atomic mass of M), which corresponds to the force constant krot with respect to the rotational motion, exhibits a positive linear correlation with energy barriers (Fig. 5c). The decreasing trend of ωrot for Te → Se → S is directly related to the dynamic stability or the phase transition between the cubic phase and the tilted orthorhombic phase (Fig. 4 and Supplementary Fig. 14). Thus, the softening of M+ ion migration (or octahedral rotational) mode could be the origin of the low energy migration energy of hydride antiperovskites.
Experimentally, we measured lithium/sodium-ion conductivity of cold-pressed M3HCh samples (M = Li, Na; Ch = S, Se, Te) using electrochemical impedance spectroscopy (EIS). As a representative example, we show in Supplementary Fig. 18 Nyquist plots of Na3HSe, featuring typical ionic impedance response with a semicircle which has a capacitance of ~10–10 F at high frequencies and a low-frequency tail. Since the bulk and grain boundary resistance cannot be specifically deconvoluted48, the total conductivity in Fig. 5d is attributed to the bulk and grain boundary resistance, which might be the reason why no clear trend in the composition dependence of ionic conductivity is observed. The activation energies obtained from the Arrhenius fit for the pristine M3HCh (Fig. 5d) are in the range of 0.44 to 0.53 eV for cubic phases and 0.30 eV for orthorhombic Na3HS, which are comparable with other superionic conductors such as garnet Li7La3Nb2O1249 and β-Li3PS450. Compared with the computed migration barrier (Fig. 5a, b), the experimentally obtained activation energy (Fig. 5d) is relatively large. The discrepancy might result from two facts: the low concentration of intrinsic charge carriers (i.e., vacancies/interstitials) which we expect in the synthesized sample, whereas the calculated migration barriers assume intrinsic vacancies or interstitials of lithium/sodium. In addition, the migration barrier is separate from defect formation energy (which we list in Supplementary Table 9), whereas they are reflected in the experimental activation energies. Moreover, the computed compositional dependence (Fig. 5c) is not observed, probably due to the non-negligible resistive grain boundary and interfacial impacts on the conductivity measurement51.
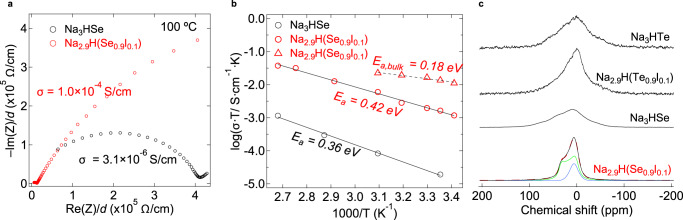
Here, we take the cubic Na3HSe compound as an example to fulfill the potential of the deformable anion matrix for fast sodium ionic diffusion. When creating a small number of sodium vacancies in Na2.9H(Se0.9I0.1), the total ionic conductivity increased to ~1 × 10–4 S/cm at 100 °C with two orders of magnitude higher than the undoped one (Fig. 6a). It is also noteworthy that the Nyquist plots of I-doped Na2.9H(Se0.9I0.1) sample at a lower temperature (Supplementary Fig. 19) show two semicircles at the high frequency where the small half-semicircle corresponds to the grain/bulk resistance and a low-frequency tail. As shown in Fig. 6b, the bulk activation energy for sodium-ion transport in Na2.9H(Se0.9I0.1) is determined as Ea,bulk = 0.18 eV, which is close to the calculated migration barrier of 0.16 eV via vacancy mechanism in Na3HSe (Fig. 5a). Detailed structural characterization of Na2.9H(Se0.9I0.1) is shown in Supplementary Fig. 20 and Supplementary Table 10.
Fig. 6. Na+ diffusion in Na3HSe and Na2.9H(Se0.9I0.1).
a Impedance plots at 100 °C of the cold-pressed pellets and the impedance is normalized to the respective pellet thickness. b Arrhenius plots of the total conductivity (circles) and bulk conductivity (triangles). c 23Na static NMR spectra measured under 7 T at room temperature. For Na2.9H(Se0.9I0.1), observed spectra (black solid), fitting spectra (red dashed), and deconvolved peak 1 with CQ = 1.30 MHz, ŋ = 0.16 (green) and peak 2 with CQ = 1.33 MHz, ŋ = 0.03 (blue).
The 23Na NMR data (Fig. 6c) exhibit a distinct behavior associated with the bulk diffusion of Na+ ions in pristine and I-doped samples. The analysis of the spectra revealed that quadrupole interaction is dominant in Na2.9H(Se0.9I0.1) reflecting the faster Na+ diffusion, whereas dipole–dipole interactions of the 23Na nuclei are non-averaged in the isostructural Na3HSe, Na3HTe, and I-doped variant52. The line width of iodine-doped sample is narrower than that of undoped one, implying that faster short-range (i.e., bulk) ion dynamics. Furthermore, the line widths of Na3HTe NMR spectra (Supplementary Fig. 21) were observed to decrease with increasing temperature (known as motional narrowing) and dipole–dipole interactions are progressively averaged due to the thermally activated motion of the Na ions21. The enhancement of Na+ conductivity we have achieved by the common aliovalent substitution approach promises the excellent potential of soft hydride–chalcogenide anion framework for Li+/Na+ diffusion. There is still much room for improvement, such as mixed halide doping in A and/or B site, in future owing to the versatility of the perovskite-based crystal structures.
Discussion
By exploiting the simple but unique hydride anion, a series of antiperovskites with the formula of M3HCh (M = Li, Na; Ch = S, Se, Te) has been successfully synthesized. Detailed structural characterizations demonstrate that the highly flexible size of hydride in the octahedral center explains the robustness of the ideal cubic structure in a wide compositional range with a 0.09 Å H–M bond difference, much longer than other antiperovskites and perovskites. Theoretically, the family of LiRAPs and NaRAPs composed by soft anion sublattices of polarizable hydride and chalcogenide are shown to have low migration barriers for Li/Na bulk transport, where the rotational phonon mode by HM6 octahedron possibly facilitates the Li/Na migration. Experimentally, high conductivity and low bulk activation energy have been demonstrated in the iodine-doped Na2.9H(Se0.9I0.1), with the total ionic conductivity of 1 × 10–4 S/cm at 100 °C. Further optimization through structural design and chemical doping would promise to achieve their full potential. The exploitation of size-flexible hydride anion developed in this work will be favorable for other ion-conducting materials, also help to induce novel states of matter and excitation phenomena.
Methods
Materials synthesis
Polycrystalline M3HCh samples (M = Li, Na; Ch = S, Se, Te) were synthesized via high-pressure solid-state reactions using MH and M2Ch as starting reagents. We used as-received LiH (Sigma-Aldrich, 99%), Li2S (Sigma-Aldrich, 99%), NaH (Sigma-Aldrich, 99%), Na2S (Sigma-Aldrich, 99%), Na2Se (Kojundo, 99%), Na2Te (Kojundo, 99%), while Li2Se and Li2Te were prepared using LiEt3BH (Sigma-Aldrich), Se (Rare Metallic Co., LTD., 99.9%) and Te (Rare Metallic Co., LTD., 99.9%). There is a tendency that excess use of alkali metal hydride improves sample purity. The starting materials were well-ground and pelletized, and the pellets were inserted into a boron nitride (BN) sleeve. The two ends of the sleeve were then sealed with BN caps. The assemble was loaded into a graphite tube heater and then enclosed in a pyrophyllite cube serving as a pressure medium. All above procedures were handled in a N2-filled glovebox due to air and moisture sensitivity of the precursors and synthesized materials. Finally, the assembly was pressed at 5 GPa, heated to 700 °C in 10 min, kept for 2 h, and cooled down to room temperature in 5 min before pressure was released. All the iodine-doped samples (e.g., Na2.9H(Se0.9I0.1), Na2.9H(Te0.9I0.1), Li2.9H(S0.9I0.1)) were successfully synthesized using NaI/LiI (Sigma-Aldrich, 99%) as iodine source under the same condition with undoped one.
Powder X-ray and neutron diffraction
We characterized the purity and crystal structures of as-prepared M3HCh samples at room temperature by powder X-ray diffraction (XRD) measurements using Rigaku Smart Lab with Cu radiation and Bruker D8 ADVANCE with Mo radiation. High-resolution synchrotron XRD experiments (SXRD) were performed at BL02B2 of SPring-8 (Japan) equipped with MYTHEN solid-state detectors. Time-of-flight(TOF) powder neutron diffraction (ND) data of Li3HS and Na3HS samples were collected on iMATERIA and SPICA diffractometers installed at the Material and Life science Facility (MLF) in the Japan Proton Accelerator Research Complex (J-PARC). The powder samples were sealed in cylindrical vanadium cells of dimensions 6 mm in diameter, 55 mm in height, and 100 μm in thickness. Rietveld refinements were performed on neutron data taken at the backscattering bank (2θ = 155° for iMATERIA and 2θ = 160.77° for SPICA). Data were evaluated and refined using the FULLPROF suite, JANA2006, and Z-Rietveld softwares53. VESTA was used to display crystal structure and to calculate geometric properties.
Impedance spectroscopy
Ionic conductivities of cold-pressed pellets of M3HCh were measured by electrochemical impedance spectroscopy (EIS) with a constant voltage of 10 mV in the frequency range of 1 MHz to 0.1 Hz using an ECS Modulab potentiostat/galvanostat. Around 80 mg of the specimen was placed between two stainless-steel rods as an ion-blocking electrode in a custom-made Swagelok cell and pressed into a 10 mm diameter pellet by a hydraulic press at 18 MPa for 1 min in an Ar-filled glovebox. For activation energy measurements, we applied a temperature loop starting from 25 °C to 100 °C. The measurements at each temperature were collected after being held for 3 h to ensure the temperature stabilization. All equivalent circuits of Nquist plots were fitted using the EC-Lab software package Z-fit.
NMR spectroscopy
23Na NMR measurements were performed using JNM-ECA300 under 7 T with the 23Na Lamor frequency of 79.5 MHz and ECA600 (JEOL) under 14 T with 158.8 MHz frequency. For magic-angle spinning (MAS) NMR, the sample was packed into a 4-mm zirconia rotor in an Ar-filled glovebox and the spinning speed was 10 kHz. For variable-temperature 23Na static NMR, the sample was sealed in an evacuated borosilicate glass tube. The 23Na chemical shifts of all spectra were referenced to 1 M NaCl aqueous solution at 0 ppm and the peaks of the spectra were deconvoluted using a Dmfit program. To assign the observed 23Na spectra, first-principles calculations based on DFT were carried out using the WIEN2k codes54,55. The muffin-tin radius, RMT, of Na, H, and Se atoms was 2.0, 1.5, and 2.0, respectively, for cubic Na3HSe. The volume optimization was performed prior to the chemical shift calculation. Self-consistent cycles were carried out at an energy convergence of 0.0001 Ry. The RMTKmax determining the number of basis function was set to 7.0, Gmax was 12, and the number of k-points in the irreducible Brillouin zone was 35. The electric-field gradient (EFG), the asymmetry parameter (ŋ), and the magnetic shielding coefficient (σiso) were calculated for the final optimized structure. To convert σiso into the chemical shift (δiso) for comparison, σiso of Na2SiO3 with δiso = 20.0 ppm56, α-Na2Si2O5 with δiso = 17.4 ppm52, and Na2SO4 with δiso = –8.5 ppm52 were calculated in the same manner.
Supplementary information
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research on Innovative Areas “Mixed Anion” (No. JP16H06439; JP16H06440; JP16H06441; JP17H05491), JSPS Core-to-Core Program (A) Advanced Research Networks (16H00888), CREST (JPMJCR1421), and JSPS KAKENHI (18H03832). The neutron experiments were conducted at J-PARC (2019A0017, 2017L1302). The synchrotron radiation experiments were performed at the BL02B2 of SPring-8, with the approval of the Japan Synchrotron Radiation Research Institute (JASRI).
Author contributions
S.H.G., T.B., and S.F. contributed equally to this work. S.H.G. and T.B. carried out all the synthetic work. T.B., C.T., and H.U. contributed to the refinement of XRD and ND data. S.F. and A.K. performed theoretical calculations. S.H.G., K.Y., Y.X., and Y.U. contributed the conductivity measurements. I.O. and H.T. contributed the NMR measurements and analysis. Y.W. contributed to the precursor preparation. K.F. and M.Y. helped ND measurement and analyses. S.H.G., S.F., C.T., and H.K. prepared the paper and figures. H.K. designed and coordinated this study, contributed to all measurements and analyses.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Anthony West and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shenghan Gao, Thibault Broux, Susumu Fujii.
Contributor Information
Cédric Tassel, Email: cedric@scl.kyoto-u.ac.jp.
Hiroshi Kageyama, Email: kage@scl.kyoto-u.ac.jp.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-20370-2.
References
- 1.Jeon NJ, et al. Compositional engineering of perovskite materials for high-performance solar cells. Nature. 2015;517:476–480. doi: 10.1038/nature14133. [DOI] [PubMed] [Google Scholar]
- 2.Yang WS, et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science. 2015;348:1234–1237. doi: 10.1126/science.aaa9272. [DOI] [PubMed] [Google Scholar]
- 3.Kamishima K, et al. Giant magnetoresistance in the intermetallic compound Mn3GaC. Phys. Rev. B. 2001;63:024426. doi: 10.1103/PhysRevB.63.024426. [DOI] [Google Scholar]
- 4.He T, et al. Superconductivity in the non-oxide perovskite MgCNi3. Nature. 2001;411:54–56. doi: 10.1038/35075014. [DOI] [PubMed] [Google Scholar]
- 5.Song X, et al. Adjustable zero thermal expansion in antiperovskite manganese nitride. Adv. Mater. 2011;23:4690–4694. doi: 10.1002/adma.201102552. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka K, Takagi H. Zero thermal expansion in a pure-form antiperovskite manganese nitride. Appl. Phys. Lett. 2009;94:131904. doi: 10.1063/1.3110046. [DOI] [Google Scholar]
- 7.Jia, X. et al. Ni3FeN nanoparticles derived from ultrathin NiFe-layered double hydroxide nanosheets: an efficient overall water splitting electrocatalyst. Adv. Energy Mater.6, 1502585 (2016).
- 8.Takahashi T, Yamamoto O. The Ag/Ag3SI/I2 solid-electrolyte cell. Electrochim. Acta. 1966;11:779–789. doi: 10.1016/0013-4686(66)87055-X. [DOI] [Google Scholar]
- 9.Lai KT, Antonyshyn I, Prots Y, Valldor M. Anti-perovskite Li-battery cathode materials. J. Am. Chem. Soc. 2017;139:9645–9649. doi: 10.1021/jacs.7b04444. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Daemen LL. Superionic conductivity in lithium-rich anti-perovskites. J. Am. Chem. Soc. 2012;134:15042–15047. doi: 10.1021/ja305709z. [DOI] [PubMed] [Google Scholar]
- 11.Lü X, et al. Antiperovskite Li3OCl superionic conductor films for solid-state Li-ion batteries. Adv. Sci. 2016;3:1500359. doi: 10.1002/advs.201500359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emly A, Kioupakis E, Van Der Ven A. Phase stability and transport mechanisms in antiperovskite Li3OCl and Li3OBr superionic conductors. Chem. Mater. 2013;25:4663–4670. doi: 10.1021/cm4016222. [DOI] [Google Scholar]
- 13.Sun Y, et al. Rotational cluster anion enabling superionic conductivity in sodium-rich antiperovskite Na3OBH4. J. Am. Chem. Soc. 2019;141:5640–5644. doi: 10.1021/jacs.9b01746. [DOI] [PubMed] [Google Scholar]
- 14.Andersen NH, Kjems JK, Hayes W. Ionic conductivity of the perovskites NaMgF3, KMgF3, KMgK3 and KZnF3 at high temperatures. Solid State Ion. 1985;17:143–145. doi: 10.1016/0167-2738(85)90063-3. [DOI] [Google Scholar]
- 15.Deng Z, Radhakrishnan B, Ong SP. Rational composition optimization of the lithium-rich Li3OCl1-xBrx anti-perovskite superionic conductors. Chem. Mater. 2015;27:3749–3755. doi: 10.1021/acs.chemmater.5b00988. [DOI] [Google Scholar]
- 16.Kim K, Siegel DJ. Correlating lattice distortions, ion migration barriers, and stability in solid electrolytes. J. Mater. Chem. A. 2019;7:3216–3227. doi: 10.1039/C8TA10989C. [DOI] [Google Scholar]
- 17.Hanghofer I, et al. Untangling the structure and dynamics of lithium-rich anti-perovskites envisaged as solid electrolytes for batteries. Chem. Mater. 2018;30:8134–8144. doi: 10.1021/acs.chemmater.8b02568. [DOI] [Google Scholar]
- 18.Ohno S, et al. Materials design of ionic conductors for solid state batteries. Prog. Energy. 2020;2:022001. doi: 10.1088/2516-1083/ab73dd. [DOI] [Google Scholar]
- 19.Smith JG, Siegel DJ. Low-temperature paddle-wheel effect in glassy solid electrolytes. Nat. Commun. 2020;11:1–11. doi: 10.1038/s41467-020-15245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen M. Volume effect or paddle‐wheel mechanism—fast alkali‐metal ionic conduction in solids with rotationally disordered complex anions. Angew. Chem. Int. Ed. 1991;30:1547–1558. doi: 10.1002/anie.199115471. [DOI] [Google Scholar]
- 21.Gao Y, et al. Classical and emerging characterization techniques for investigation of ion transport mechanisms in crystalline fast ionic conductors. Chem. Rev. 2020;120:5954–6008. doi: 10.1021/acs.chemrev.9b00747. [DOI] [PubMed] [Google Scholar]
- 22.Bachman JC, et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 2016;116:140–162. doi: 10.1021/acs.chemrev.5b00563. [DOI] [PubMed] [Google Scholar]
- 23.Kraft MA, et al. Influence of lattice polarizability on the ionic conductivity in the lithium superionic argyrodites Li6PS5X (X = Cl, Br, I) J. Am. Chem. Soc. 2017;139:10909–10918. doi: 10.1021/jacs.7b06327. [DOI] [PubMed] [Google Scholar]
- 24.He X, Zhu Y, Mo Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 2017;8:15893. doi: 10.1038/ncomms15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krauskopf T, et al. Comparing the descriptors for investigating the influence of lattice dynamics on ionic transport using the superionic conductor Na3PS4-xSex. J. Am. Chem. Soc. 2018;140:14464–14473. doi: 10.1021/jacs.8b09340. [DOI] [PubMed] [Google Scholar]
- 26.Muy S, et al. Tuning mobility and stability of lithium ion conductors based on lattice dynamics. Energy Environ. Sci. 2018;11:850–859. doi: 10.1039/C7EE03364H. [DOI] [Google Scholar]
- 27.Wang Y, et al. Design principles for solid-state lithium superionic conductors. Nat. Mater. 2015;14:1026–1031. doi: 10.1038/nmat4369. [DOI] [PubMed] [Google Scholar]
- 28.Di Stefano D, et al. Superionic diffusion through frustrated energy landscape. Chem. 2019;5:2450–2460. doi: 10.1016/j.chempr.2019.07.001. [DOI] [Google Scholar]
- 29.Hanghofer I, Gadermaier B, Wilkening HMR. Fast rotational dynamics in argyrodite-type Li6PS5X (X: Cl, Br, I) as seen by 31P nuclear magnetic relaxation on cation-anion coupled transport in thiophosphates. Chem. Mater. 2019;31:4591–4597. doi: 10.1021/acs.chemmater.9b01435. [DOI] [Google Scholar]
- 30.Kweon KE, et al. Structural, chemical, and dynamical frustration: origins of superionic conductivity in closo-borate solid electrolytes. Chem. Mater. 2017;29:9142–9153. doi: 10.1021/acs.chemmater.7b02902. [DOI] [Google Scholar]
- 31.Krauskopf T, Pompe C, Kraft MA, Zeier WG. Influence of lattice dynamics on Na+ transport in the solid electrolyte Na3PS4-xSex. Chem. Mater. 2017;29:8859–8869. doi: 10.1021/acs.chemmater.7b03474. [DOI] [Google Scholar]
- 32.Kamaya N, et al. A lithium superionic conductor. Nat. Mater. 2011;10:682–686. doi: 10.1038/nmat3066. [DOI] [PubMed] [Google Scholar]
- 33.Kageyama H, et al. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018;9:772. doi: 10.1038/s41467-018-02838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohtadi R, Orimo SI. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2016;2:16091. doi: 10.1038/natrevmats.2016.91. [DOI] [Google Scholar]
- 35.Kohlmann H, Skripov AV, Soloninin AV, Udovic TJ. The anti-perovskite type hydride InPd3H0.89. J. Solid State Chem. 2010;183:2461–2465. doi: 10.1016/j.jssc.2010.08.015. [DOI] [Google Scholar]
- 36.Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A. 1976;32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 37.Nuss J, Mühle C, Hayama K, Abdolazimi V, Takagi H. Tilting structures in inverse perovskites, M3TtO (M = Ca, Sr, Ba, Eu; Tt = Si, Ge, Sn, Pb) Acta Crystallogr. B. 2015;71:300–312. doi: 10.1107/S2052520615006150. [DOI] [PubMed] [Google Scholar]
- 38.Goldschmidt VM. Die Gesetze der Krystallochemie. Naturwissenschaften. 1926;14:477–485. doi: 10.1007/BF01507527. [DOI] [Google Scholar]
- 39.Masuda N, et al. Hydride in BaTiO2.5H0.5: a labile ligand in solid state chemistry. J. Am. Chem. Soc. 2015;137:15315–15321. doi: 10.1021/jacs.5b10255. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita H, et al. Chemical pressure-induced anion order-disorder transition in LnHO enabled by hydride size flexibility. J. Am. Chem. Soc. 2018;140:11170–11173. doi: 10.1021/jacs.8b06187. [DOI] [PubMed] [Google Scholar]
- 41.Broux T, et al. High-pressure polymorphs of LaHO with anion coordination reversal. J. Am. Chem. Soc. 2019;141:8717–8720. doi: 10.1021/jacs.9b03320. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi Y, Tsujimoto Y, Kageyama H. Property engineering in perovskites via modification of anion chemistry. Annu. Rev. Mater. Res. 2018;48:303–326. doi: 10.1146/annurev-matsci-070317-124415. [DOI] [Google Scholar]
- 43.Brese NE, O’Keeffe M. Bond‐valence parameters for solids. Acta Crystallogr. B. 1991;47:192–197. doi: 10.1107/S0108768190011041. [DOI] [Google Scholar]
- 44.Tang W, Sanville E, Henkelman G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter. 2009;21:84204. doi: 10.1088/0953-8984/21/8/084204. [DOI] [PubMed] [Google Scholar]
- 45.Hinuma Y, Grüneis A, Kresse G, Oba F. Band alignment of semiconductors from density-functional theory and many-body perturbation theory. Phys. Rev. B. 2014;90:155405. doi: 10.1103/PhysRevB.90.155405. [DOI] [Google Scholar]
- 46.Cancellieri C, et al. Polaronic metal state at the LaAlO3/SrTiO3 interface. Nat. Commun. 2016;7:10386. doi: 10.1038/ncomms10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadano, T. & Tsuneyuki, S. Self-consistent phonon calculations of lattice dynamical properties in cubic SrTiO3 with first-principles anharmonic force constants. Phys. Rev. B92, 054301 (2015).
- 48.Irvine JTS, Sinclair DC, West AR. Electroceramics: characterization by impedance spectroscopy. Adv. Mater. 1990;2:132–138. doi: 10.1002/adma.19900020304. [DOI] [Google Scholar]
- 49.Thangadurai V, Kaack H, Weppner WJF. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta) J. Am. Ceram. Soc. 2003;86:437–440. doi: 10.1111/j.1151-2916.2003.tb03318.x. [DOI] [Google Scholar]
- 50.Liu Z, et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013;135:975–978. doi: 10.1021/ja3110895. [DOI] [PubMed] [Google Scholar]
- 51.Dawson JA, Canepa P, Famprikis T, Masquelier C, Islam MS. Atomic-scale influence of grain boundaries on Li-ion conduction in solid electrolytes for all-solid-state batteries. J. Am. Chem. Soc. 2018;140:362–368. doi: 10.1021/jacs.7b10593. [DOI] [PubMed] [Google Scholar]
- 52.Koller H, Engelhardt G, Kentgens APM, Sauer J. 23Na NMR spectroscopy of solids: interpretation of quadrupole interaction parameters and chemical shifts. J. Physial Chem. 1994;98:1544–1551. doi: 10.1021/j100057a004. [DOI] [Google Scholar]
- 53.Oishi R, et al. Rietveld analysis software for J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 2009;600:94–96. doi: 10.1016/j.nima.2008.11.056. [DOI] [Google Scholar]
- 54.Oikawa I, Takamura H. 45Sc NMR spectroscopy and first-principles calculation on the symmetry of ScO6 polyhedra in BaO-Sc2O3-based oxides. Dalt. Trans. 2014;43:9714–9721. doi: 10.1039/c4dt00426d. [DOI] [PubMed] [Google Scholar]
- 55.Blaha P, et al. WIEN2k: an APW+lo program for calculating the properties of solids. J. Chem. Phys. 2020;152:074101. doi: 10.1063/1.5143061. [DOI] [PubMed] [Google Scholar]
- 56.Jones AR, Winter R, Neville Greaves G, Smith IH. 23Na, 29Si, and 13C MAS NMR investigation of glass-forming reactions between Na2CO3 and SiO2. J. Phys. Chem. B. 2005;109:23154–23161. doi: 10.1021/jp053953y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.