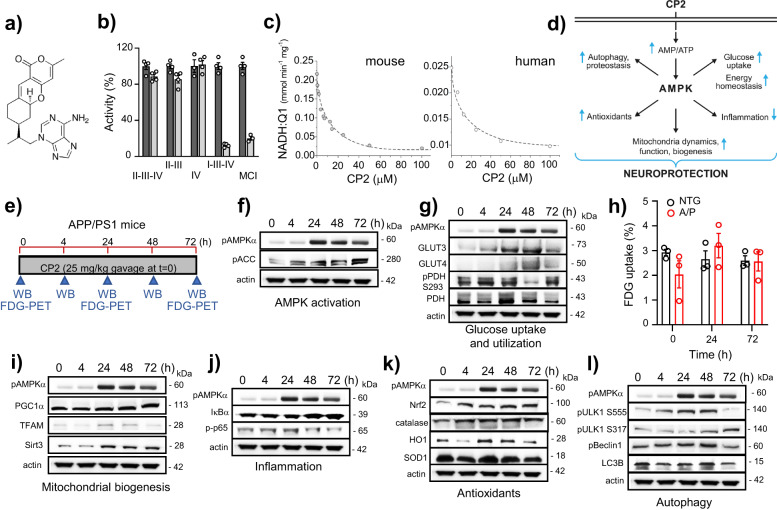
Fig. 1. Acute CP2 treatment activates multiple AMPK-dependent neuroprotective mechanisms in symptomatic APP/PS1 mice improving energy homeostasis in the brain.
a CP2 structure. b CP2 (light gray bars, 50 µM) does not affect the activity of succinate oxidase (complexes II-III-IV), succinate:cytochrome c reductase (complexes II-III), and ferrocytochrome c oxidase (complex IV only), but significantly inhibits MCI affecting NADH oxidase (complexes I-III-IV) and NADH:ubiquinone (complex I only) in mouse brain mitochondria. Vehicle-dark gray bars. c CP2 inhibits MCI in mitochondria isolated from the mouse and postmortem human cortical tissue. d Neuroprotective pathways activated by CP2 in the brain converge on AMPK activation. e Timeline of acute CP2 administration via gavage (25 mg/kg) to APP/PS1 mice 9–10-month-old. Brain tissues from 1 mouse per each time point were examined using western blot analysis. Independent cohort of mice was subjected to the in vivo FDG-PET (n = 3 mice per group). f–l Western blot analysis of brain tissue from acutely gavaged APP/PS1 mice from e confirms that CP2 activates multiple AMPK-dependent neuroprotective mechanisms in symptomatic APP/PS1 mice. h Untreated symptomatic APP/PS1 mice 9–10-month-old have reduced glucose uptake in the brain determined using in vivo FDG-PET compared to NTG littermates (time 0 h). Acute CP2 oral administration improves glucose uptake brining glucose levels in APP/PS1 mice to the same level as in untreated age-matched and sex-matched NTG mice (time 24 and 72 h); n = 3 mice per group.