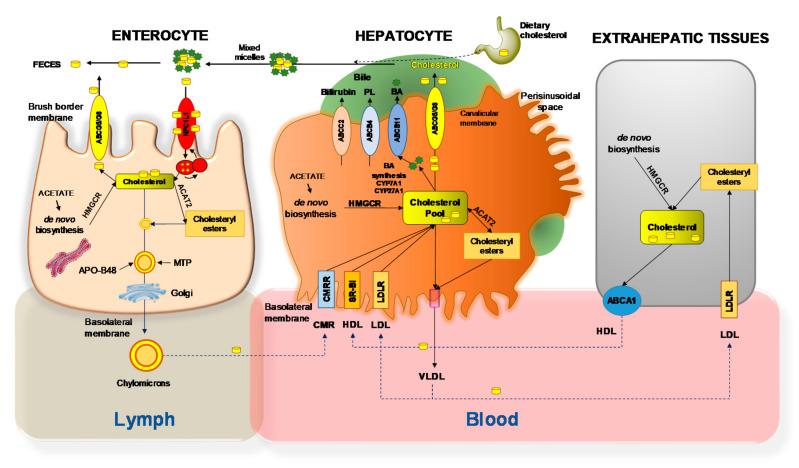
Figure 2.
Net cholesterol flow through the body. In the small intestinal lumen, micellar solubilization facilitates the diffusion of sterols through enterocyte. Mixed micelles (Bile acids (BA) and cholesterol) increase the uptake of cholesterol via the Niemann–Pick C1 like 1 (NPC1L1) protein, a sterol influx transporter. The transporter ABCG5/G8 (brush border membrane) promotes active efflux of cholesterol from the enterocyte back into the intestinal lumen for fecal excretion. The combined regulation of NPC1L1 and ABCG5/G8 modulates the amount of cholesterol reaching the lymph from the intestine. Cholesterol (either absorbed or newly synthesized molecules from acetate by 3-hydroxy-3-methylglutaryl-CoA reductase [HMGCoAR]) are esterified to fatty acids by acyl-CoA:cholesterol acyltransferase isoform 2 (ACAT2), to form cholesteryl esters. This pool of lipids contributes to the assembly of chylomicrons, which are enriched with the apolipoprotein B-48 (apoB-48) and require microsomal triglyceride transfer protein (MTTP). The core of chylomicrons secreted in lymph contains triglycerides and cholesteryl esters. The surface contains phospholipids, unesterified cholesterol, and apolipoproteins such as apoB-48, apoA-I, and apoA-IV [24,25]. The hepatic uptake of cholesterol involves the scavenger receptor class B type I (SR-BI) for high-density lipoprotein (HDL), the low-density lipoprotein (LDL) receptor (LDLR) for LDL, and the chylomicron remnant receptor (CMRR) for chylomicron remnants (CMR). Biosynthesis of hepatic cholesterol (CH) from acetate requires the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR). Most of the cholesterol undergoes the synthesis of BA involving two rate-limiting enzymes in the classical pathway (cholesterol 7a-hydroxylase, CYP7A1) and the alternative pathway (sterol 27-hydroxylase, CYP27A1). Some cholesterol is esterified by acyl-coenzyme A: cholesterol acyltransferase isoform 2 (ACAT2). This step provides lipid droplets serving for storage within the hepatocytes. Some of the cholesterol is arranged with the very-low-density lipoprotein (VLDL), secreted into blood. Cholesterol secretion across the canalicular membrane of hepatocytes into the bile canaliculus requires the specific ATP-binding cassette transporter ABCG5/G8. This process is paralleled by secretion of the two other lipids acting as cholesterol transporters in bile, i.e., BA via ABCB11, and phospholipids (PL) via ABCB4. In extrahepatic tissues VLDL delivers cholesterol across LDL and LDLR, while cholesterol is excreted via the transporter ABCA1 and formation of HDL. Abbreviations: CYP7A1, cholesterol 7-α; CYP27A1, sterol 27-hydroxylase; RER, rough endoplasmic reticulum. Adapted from D. Q-H. Wang et al. [26,27,28].