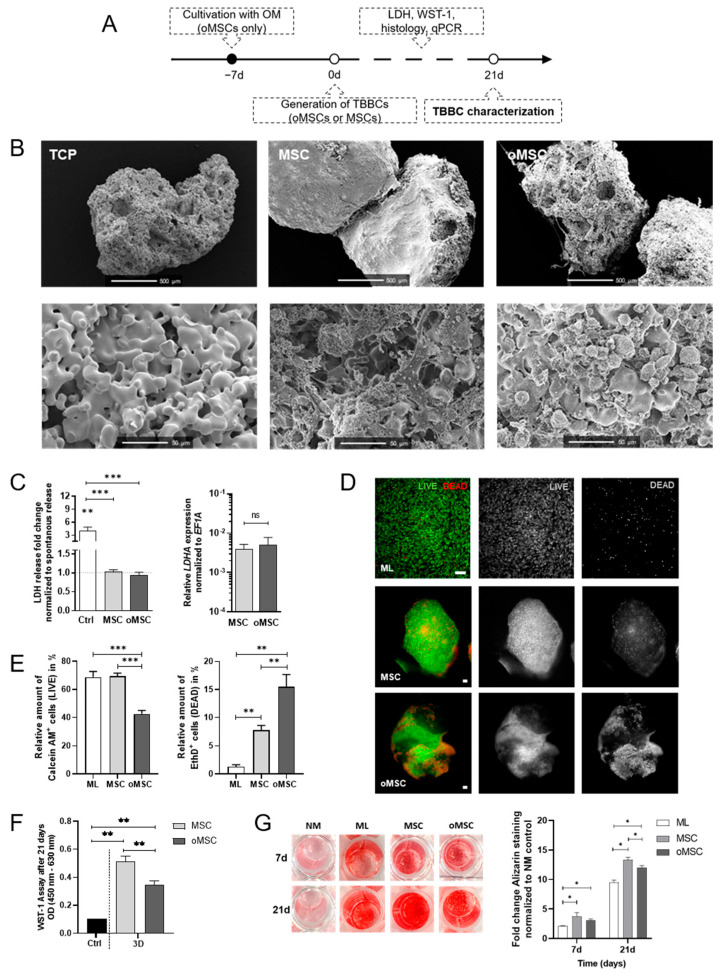
Figure 2.
In vitro studies on β-TCP biocompatibility and cell survival comparing the suitability of human mesenchymal stromal cells (MSCs) and osteogenic pre-differentiated MSCs (oMSCs). (A) Experimental design of the in vitro TCP-based bone component (TBBC). (B) Structural evaluation of β-TCP using scanning electron microscopy. Exemplary images of n = 6. Scale bars show 500 µm and 50 µm as indicated in the images. (C) LDH-assay was conducted after 24 h to confirm the biocompatibility of β-TCP. Ctrl = 2% of Triton X‑100. Data are shown as mean ± SEM for n = 10–12. Gene expression of lactate dehydrogenase A (LDHA) was determined by qPCR and normalized to the housekeeper gene EF1A. Data are shown as mean ± SEM for n = 4. Mann-Whitney U-test was used to determine the statistical significance between groups and Wilcoxon signed-ranked test for the spontaneous LDH release control. (D) LIVE/DEAD staining was performed after 21 days and (E) quantified using ImageJ. As control MSCs in monolayer (ML) were stained. Green and red colors discriminated between living and dead cells (scale bar = 100 µm). Representative images are shown accordingly for n = 4–6. Data are shown as mean ± SEM. (F) WST-1 assay was conducted to confirm metabolically active cells after 21 days of 3D cultivation. Ctrl = 2% of Triton X-100. Data are shown as mean ± SEM for n = 6. Mann-Whitney U-test was used. (G) MSCs and oMSCs were cultivated for 7 and 21 days in normal medium (NM control), in osteogenic medium without β‑TCP (ML) and with β‑TCP populated with MSCs (MSC) or pre-differentiated MSCs (oMSC). Alizarin Red staining was quantified (562 nm). Data are shown as mean ± SEM for n = 5. Wilcoxon matched-pairs signed rank test was used to determine the statistical significance. p-values are indicated in the graphs with * p < 0.05, ** p < 0.01 and *** p < 0.001 (ns = not significant). TCP, tricalcium phosphate; LDH, lactate dehydrogenase; EF1A, eukaryotic translation elongation factor 1 alpha.