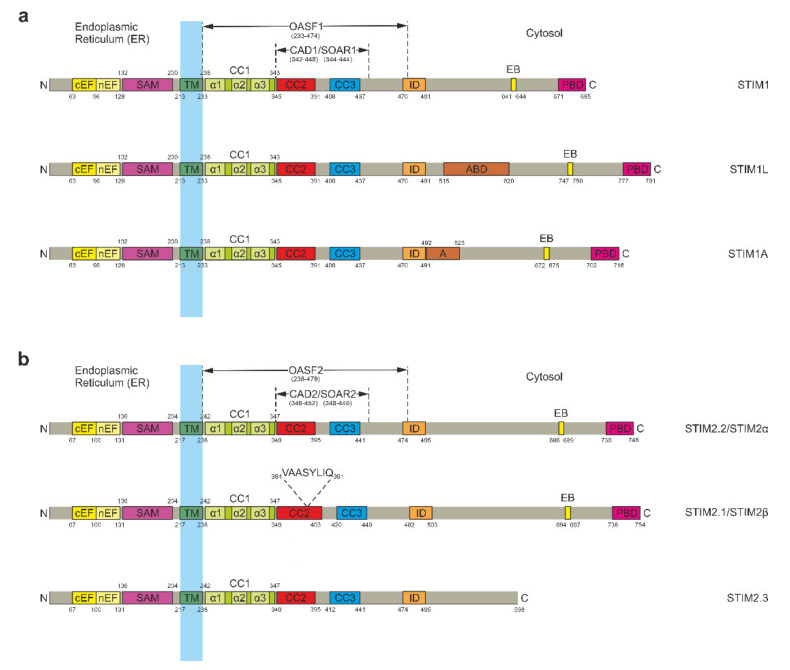
Figure 1.
Domain structure of stromal interaction molecule (STIM) proteins. (a) Primary structure of STIM1 and its isoforms STIM1 Long (STIM1L) and STIM1A. Functionally relevant domains within the endoplasmic reticulum (ER) luminal portion include the canonical (cEF) and noncanonical (nEF) EF hands as well as the sterile alpha motif (SAM). Downstream of the transmembrane domain (TM), the cytosolic portion contains three coiled coil (CC) domains commonly known as CC1, CC2, and CC3 with CC1 being further subdivided into α1, α2, and α3. The C-terminal fragment spanning all three CC domains is termed Orai-activating small fragment (OASF). Another fragment comprising CC2 and CC3 is named CRAC-activating domain (CAD) or STIM-Orai-activating region (SOAR). Further C-terminal domains include the inactivation domain (ID or ID-STIM), the microtubule end-binding domain (EB), and the polybasic domain (PBD) at the outermost C-terminus. STIM1L and STIM1A feature the same general structure but each harbor an additional C-terminal domain inserted downstream of the ID domain by alternative splicing. STIM1L thereby includes an actin-binding domain (ABD) while STIM1A possesses an insert designated as domain A. (b) Primary structure of STIM2.2/STIM2α and its isoforms STIM2.1/STIM2β and STIM2.3. For reasons of simplicity, the 87 amino acid N-terminal signal peptide insertion of STIM2.2 and its isoforms was omitted from this display [43,44,45]. Due to the high degree of similarity between STIM1 and STIM2.2, their functional domains are essentially equivalent. Alternative splicing leads to inclusion of a small 8 amino acid insert (VAASYLIQ) within the CC2 domain of STIM2.1 and to an upstream end of translation in case of STIM2.3, shortening the protein by 148 amino acids. Otherwise, both STIM2.1 and STIM2.3 correspond to STIM2.2.