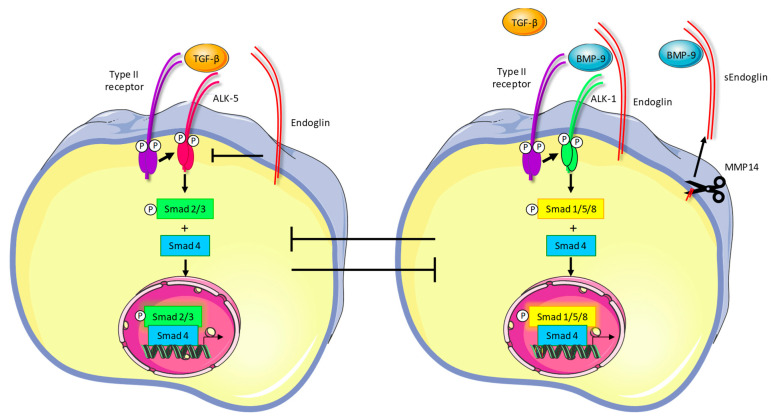
Figure 1.
Endoglin signaling. When TGF-β binds to its type-II receptor in the absence of endoglin, the type-I receptor ALK-5 is recruited and transphosphorylated. This subsequently leads to phosphorylation of Smad2/3, which then forms a complex with Smad4, translocates to the nucleus and regulates target genes involved in vessel maturation. The presence of endoglin on the cell membrane inhibits this signaling pathway (left panel). In the presence of endoglin, binding of BMP-9 or TGF-β to the type-II receptor results in recruitment and transphosphorylation of ALK-1, which phosphorylates Smad1/5/8, resulting in regulation of angiogenic target genes (right panel). Endoglin can be cleaved from the cell membrane by MMP-14, creating a soluble form of the receptor which may function as a ligand trap.