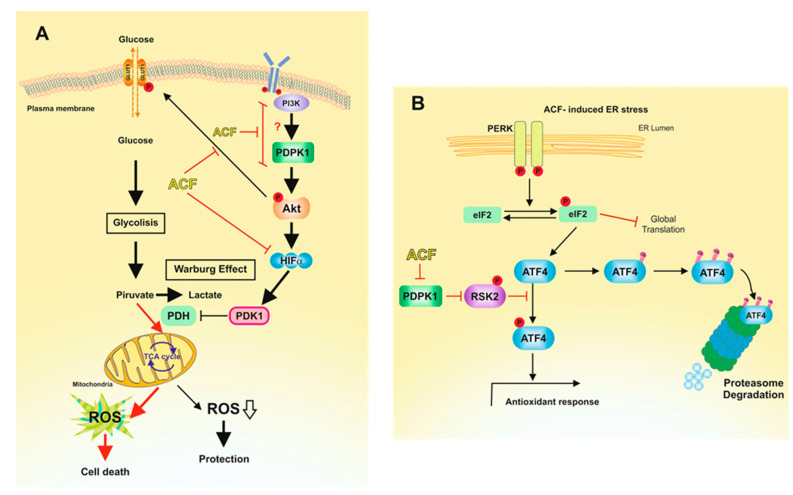
Figure 6.
Proposed mechanisms for the action of ACF on melanoma cells under normoxic conditions. (A) This picture reproduces the adaptation of melanoma cells to physiological concentrations of glucose. Restriction of glucose availability to physiological concentrations induces the production of ROS [16], which activate HIF-1α [46]. Activated HIF-1α induces glycolysis upregulation in cancer cells, a phenomenon known as the Warburg effect [47]. Thus, by increasing the conversion of pyruvate to lactate, the Warburg effect reduces ROS production by the mitochondrial OXPHOS. Activated AKT pathway contributed to glucose transport through GLUT1 plasmatic membrane translocation and activation of HIF-1α. ACF, by inhibiting HIF-1α, impedes PDK1 transcription, resulting in enhanced ROS production. In this metabolic scenario, ACF blocks the PI3K/PDPK1 pathway, resulting in impaired phosphorylation of AKT. Consistently with our results (Figure 2A), inhibition of AKT phosphorylation by ACF would also result in reduced expression of HIF-1α [31] under normoxic conditions. Red arrows indicate favored pathways in the presence of ACF. (B) Increased ROS levels induces ER stress, leading to the UPR [48]. Although ACF induces the phosphorylation of eIF2α, this is not traduced in elevated expression of ATF4. Since protein stability of ATF4 is dependent of an operative PDPK1/RSK2 pathway [29], ACF induces the destabilization of ATF4 and, therefore, the inactivation of ATF4-adaptative pathways.