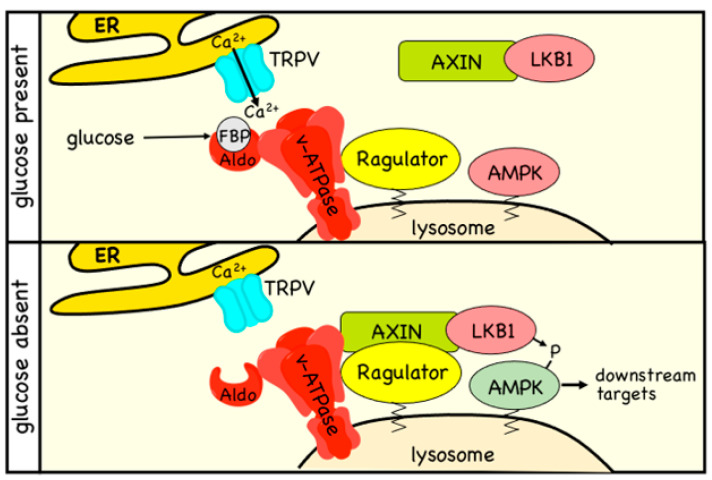
Figure 3.
Non-canonical mechanism of activation of AMPK by glucose starvation. The model is based on that in Li et al. [60]. In the presence of high glucose (top panel), a large proportion of aldolase (Aldo), which is bound to the v-ATPase proton pump on the lysosome, will have its substrate, the glycolytic intermediate fructose-1,6-bisphosphatase (FBP), bound to it. This causes the opening of TRPV Ca2+ channels located at ER:lysosome contact sites, maintaining the function of the v-ATPase and promoting mTORC1 activity via the Ragulator complex, which is anchored on the surface of the lysosome via lipid modifications. When glucose is absent or limiting (lower panel), aldolase will not be fully occupied by FBP, and it interacts with and inhibits the neighbouring TRPV channels. The v-ATPase is now inhibited, allowing an interaction between the Ragulator and the cytoplasmic Axin:LKB1 complex, and bringing LKB1 into the proximity of AMPK, which is then phosphorylated and activated. In this version of the model, a pool of AMPK is viewed as being permanently located at the lysosome due to the N-terminal myristoylation of the β subunit.