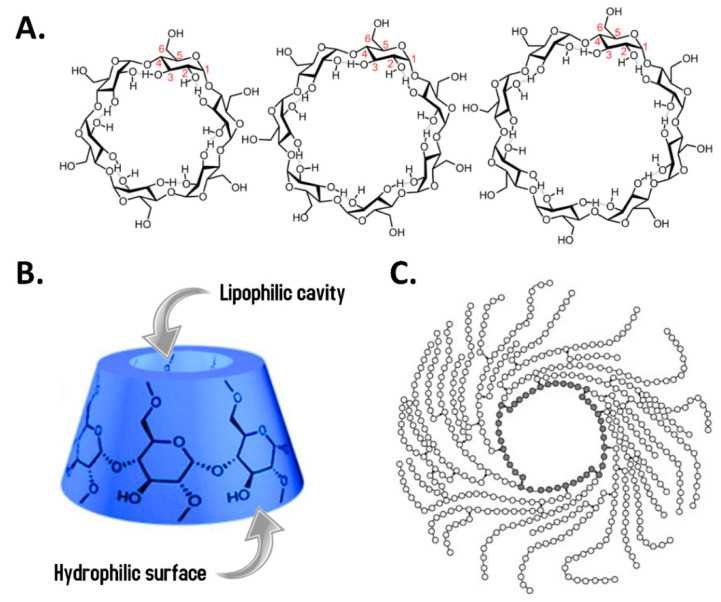
Figure 4.
Molecular structures of common cyclodextrins and schematic representation of highly branched cyclic dextrin (HBCD). (A). Chemical structure of the α-, β- and γ-cyclodextrins, which contain 6, 7 or 8 glucose units, respectively. Image taken and modified from [86]. (B). The conical shape representation of cyclodextrins. These molecules have a truncated cone shape with a hydrophilic external surface (due to the hydroxyl groups on the C2, C3 and C6 atoms) and a hydrophobic cavity (due to the inner hydrogens (H3 and H5) pointing inward) [87]. Cyclodextrins have different water solubility: α- and γ-cyclodextrins have a relatively high solubility (145 and 232 g·L−1), whereas the β-type is much less soluble in water (18.5 g·L−1). Image taken and modified from [88]. (C). The schematic representation of HBCD is shown. Open circles represent glucose units while grey-filled circles are those conforming the main centered ring. Lines and arrows are α-(1→4) and α-(1→6) glycosidic bonds, respectively. Image taken from [89].