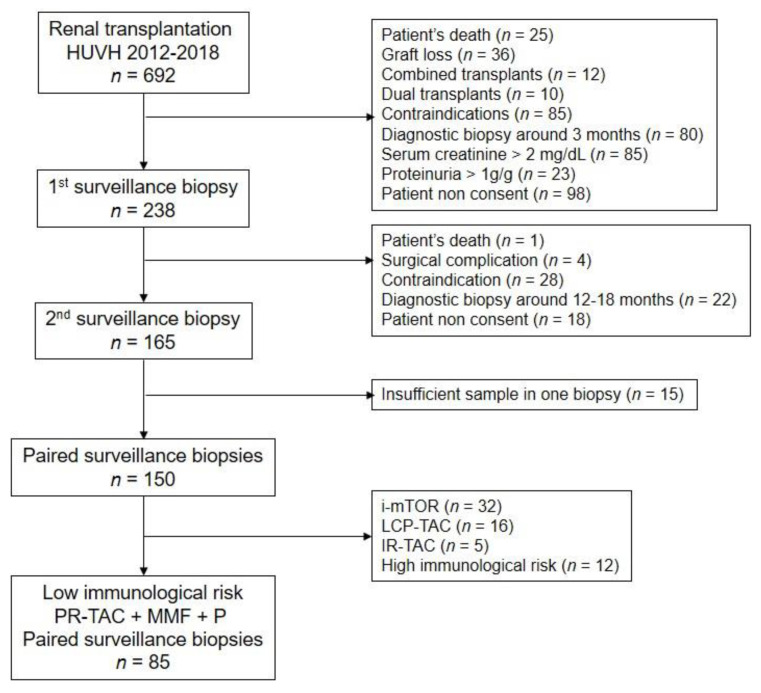
Figure 2.
Flow chart of included patients. PR-TAC, prolonged-release tacrolimus; MMF, mycophenolate mofetil; P, prednisone; LCP-TAC, extended-release tacrolimus; IR-TAC, immediate-release tacrolimus; i-mTOR, inhibitors of mammalian target of rapamycin. Contraindications for the first surveillance biopsy include treatment with oral anticoagulants (n = 33), large abdominal obesity (n = 26), technical difficulties due to perirenal hematoma or lymphocele (n = 9), idiomatic barrier (n = 13), and horseshoe kidneys (n = 4). Indications for the use of mTOR inhibitors in this cohort was as follows: inclusion in a clinical trial containing i-mTOR de novo or early conversion (n = 16), polyoma BK viremia during follow up (n = 6), CMV viremia after prophylaxis in high-risk recipients (n = 8) and skin cancer (n = 2). High immunological risk patients were defined as those with HLA donor-specific antibodies at the time of transplant (n = 7) or receiving a desensitization treatment before transplant (n = 5).