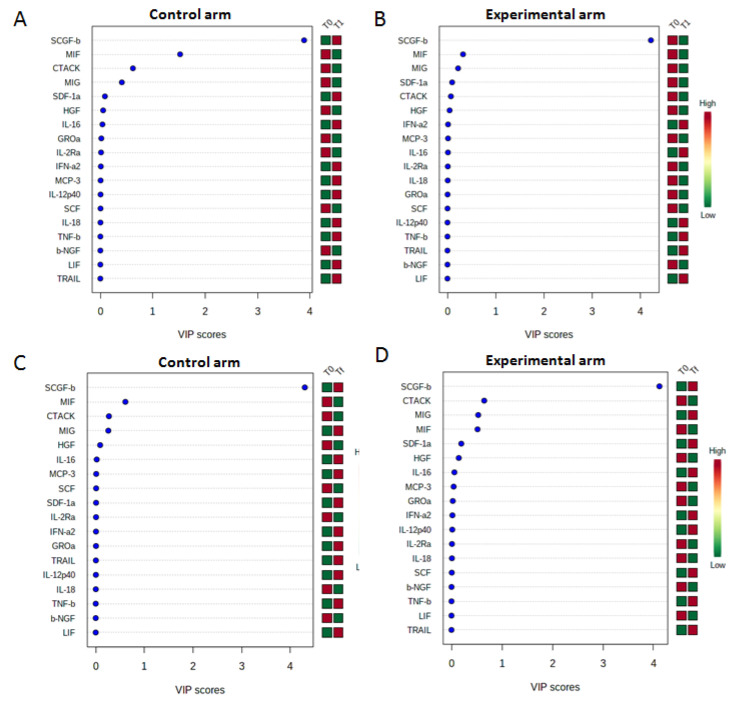
Figure 1.
Partial least squares discriminant analysis (PLS-DA) was performed to compare the cytokine concentrations on sera from Breast Cancer Patients enrolled in the randomized Mind-Body Transformations Therapy (MBT-T) study. In particular, we show variable important plot (VIP) related to the comparison between cytokines concentrations evaluated by Bio-Plex Pro Human Cytokine 21-Plex Immunoassay on sera from 7 and 16 Breast Cancer Patients enrolled in control and experimental arm, respectively, at baseline (T0) and after 1 h of the first treatment (T1) (A,B) and at baseline (T0) and the end of the MBT-T treatment (Tf) (C,D). The shown cytokines are interleukin-1α (IL-1α), interleukin-2Rα (IL-2Rα), interleukin-3 (IL-3), interleukin-12 (IL-12), interleukin-16 (IL-16), interleukin-18 (IL-18), cutaneous T cell-attracting chemokine (CTACK), Growth-regulated protein alpha (GRO-α), hepatocyte growth factor (HGF), interferon-α2 (IFN-α2), Leukemia inhibitory factor (LIF), Monocyte chemotactic protein-3 (MCP-3), Macrophage migration inhibitory factor (MIF), monokine induced by gamma interferon (MIG), beta nerve growth factor (β-NGF), colony-stimulating factor (SCF), stem cell growth factor-beta (SCGF-β), stromal cell-derived factor 1alpha (SDF-1α), tumor necrosis factor-beta (TNF-β) and Tumor necrosis factor (TNF)-Related Apoptosis Inducing Ligand (TRAIL).