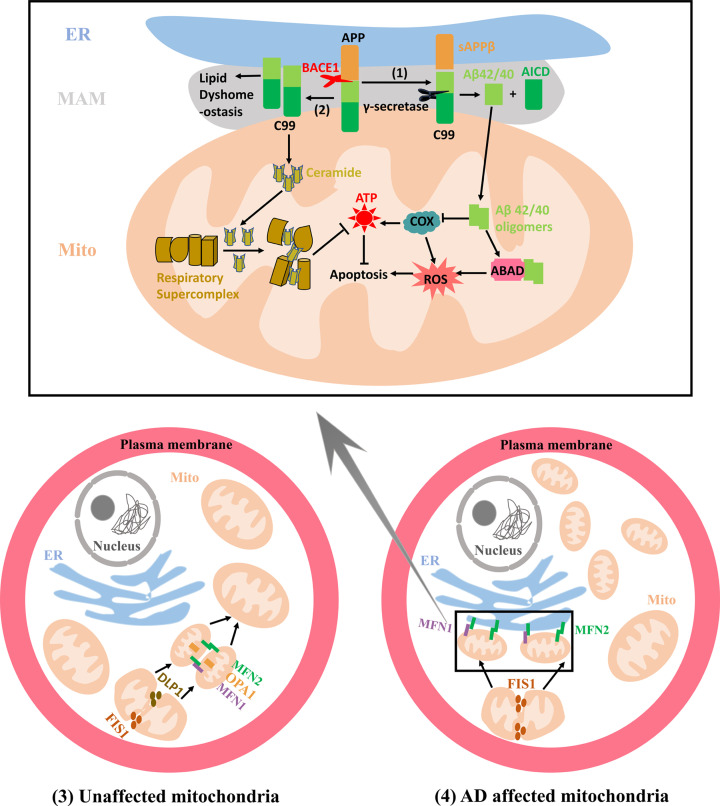
Figure 2. Mitochondrial alterations related to MAMs in AD.
(1) The increased cleavage of C99 by γ-secretase localized at the MAMs promotes Aβ42/40 generation, which are subsequently transferred to mitochondria to form mitochondrial Aβ deposition. Aβ oligomers can inhibit COX and ABAD functions, increasing the production of ROS and ultimately leading to the apoptosis. (2) C99 generated at the MAMs can affect the lipid composition in the MAMs and augment the mitochondrial ceramide concentration by activating the sphingolipid turnover and hydrolysis, which disturbs the assembly and activity of respiratory supercomplexes, ultimately resulting in bioenergetic deficiencies. (3) The mitochondrial fission can be mediated by proteins DLP1 and FIS1. The OMM fusion is regulated by proteins MFN1 and MFN2 that can form MFN1 and MFN2 homo-oligomeric or hetero-oligomeric complexes while IMM fusion respectively utilizes OPA1. In unaffected mitochondria, the mitochondria with relatively equal morphology are uniformly distributed in the cytosol due to the dynamic equilibrium between the mitochondrial fission and fusion processes. (4) In AD affected mitochondria, augmented mitochondrial fission leads to more fragmented mitochondria that tend to form a ‘ring’ around the nucleus. MFN2 is also localized at the ER and the ER MFN2 binds to mitochondrial MFN1 and MFN2 increasing the formation of the interconnection between the ER and mitochondria. DLP1, dynamin-like protein 1; FIS1, fission 1; OMM, outer mitochondrial membrane; OPA1, optic atrophy protein 1; IMM, inner mitochondrial matrix; AD, Alzheimer’s disease; ER, endoplasmic reticulum; MFN1, Mitofusin-1; MFN2, Mitofusin-2; COX, cytochrome c oxidase; Aβ, β-amyloid; MAM, Mitochondria-Associated Membrane; APP, amyloid precursor protein; BACE1, β-secretase; ABAD, alcohol dehydrogenase; ATP, adenosine triphosphate.