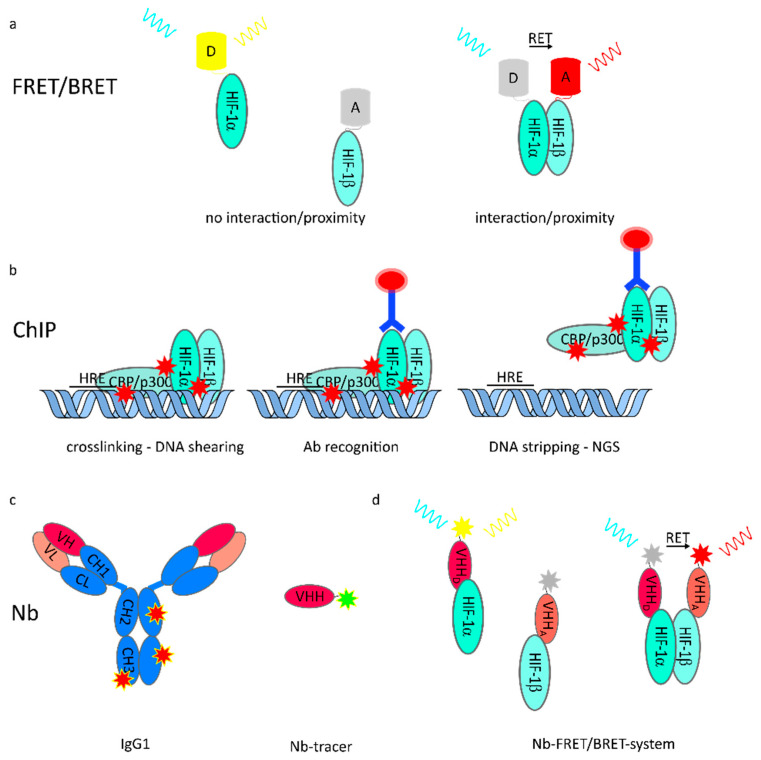
Figure 3.
Methods to unravel effects on HIF protein stability, interaction, and function in vitro and in vivo. (a) Interaction of protein (complexes) is detectable by proximity-depended resonance energy transfer such as fluorescence resonance energy transfer (FRET) or bioluminescence resonance energy transfer (BRET). Protein localization and proximity are directly imaged in vitro or in vivo and recorded with high spatiotemporal resolution; (b) Transcriptional activity of transcription factor is assessed by chromatin immunoprecipitation (ChIP). Precise antibody recognition and accuracy is mandatory for qualitative evaluation of changes upon any perturbation; (c) Nanobodies (Nb), shown in relation to conventionally used IgG1, can be functionalized and used as intravital tracers. Due to their small size, penetration depth of cells and tissue is superior. Stoichiometric labeling of Nb enables quantitative imaging; (d) Nb tracer addressing interaction partners allow in vivo FRET/BRET independent of genetic manipulation, giving rise to a multitude of new life cell/intravital interaction studies.