Abstract
Amyloid deposits in the liver are recognized by their H&E findings, consisting of acellular eosinophilic deposits in various compartments of the liver parenchyma, including the stroma, vessels, and rarely the hepatocytes. H&E findings that suggest amyloid are then confirmed by Congo red stains and subtyped when clinically needed. Two cases are reported with sinusoidal deposits of acellular material that closely mimicked amyloid on H&E, but were Congo red negative. Mass spectrometry-based proteomic analysis identified the material as fibronectin. In one case, the deposits were located in the sinusoids of a well differentiated hepatocellular carcinoma and in one case in the sinusoids of a benign liver.
Introduction
Most amyloid deposits in the liver have a similar morphological appearance to those seen elsewhere in the body, with dense acellular deposits of eosinophilic material. Most cases of liver amyloidosis are of AL type and commonly show portal and sinusoidal deposits. Less common patterns include amyloid deposition limited to arteries, mass form deposits called amyloidomas,1 and LECT2 amyloidosis, which has a globular pattern of amyloid deposition.2 Regardless of the type of amyloid or the pattern of deposition, Congo red stains are used to confirm the diagnosis. Amyloid subtypes can be determined by mass spectrometry.3 We report two cases with fibronectin deposition in the liver, both showing H&E findings that closely mimicked amyloid.
Material and Methods
Case #1 was received in consultation at Mayo Clinic Rochester. Mass spectrometry was used to determine the type of proteins deposited in the tissue.3 After the index case was examined, the pathology data base at Mayo Clinic was searched to identify additional cases of fibronectin deposition in the liver, including a search of hepatocellular carcinoma. All liver cases in our institutional and consultation archives, including core needle biopsies, resection and explants were searched for “fibronectin” for the time period January 1, 2000 to December 31, 2019. We also searched our mass spectrometry amyloid typing database for “fibronectin”. This search identified 10,654 data files involving specimens from different organ systems, including kidney, liver, heart, skin, lung, small and large bowel, eye, adipose tissue, and bone marrow etc. Of 10,654 data files, we identified an additional case of non-neoplastic liver with fibronectin deposition, case #2.
Results
Case # 1
Clinical history
An 82 year old woman was found to have a large solitary liver mass. She had a known history of diabetes mellitus, hypertension and dyslipidemia, and a newly diagnosed endometrial adenocarcinoma. On abdominal MRI, the hepatic mass measured 8 cm. The mass was identified at the junction of the right hepatic lobe, medial segment of the left hepatic lobe and caudate lobe. The lesion was solid and variably enhancing, with multiple regions of vascular flow and multiple regions of lesser enhancement. The radiological differential diagnosis was broad and included hepatocellular carcinoma, metastatic disease, focal nodular hyperplasia, hepatic adenoma and other etiologies. There was no hepatomegaly and features of portal hypertension. The patient had no history of liver disease, chronic renal disease, hematolymphoid disorders, or plasma cell dyscrasia. Liver function tests and alpha-fetoprotein levels were within normal limits. Blood urea nitrogen (26 mg/dl) and creatinine (1.3 mg/dL) levels were mildly elevated.
Histologic features
The patient underwent two needle core biopsies; the first biopsy was not diagnostic due to a paucity of tumor cells, with the biopsy mostly showing abundant extracellular homogenous eosinophilic/hyalinized material, with only scant interspersed epithelial cells, growing in atrophic cord-like and lace-like growth patterns (Figure 1A and 1B). The tumor cells had scant eosinophilic granular cytoplasm, and low grade cytological atypia. The second biopsy showed similar findings, with striking amounts of extracellular eosinophilic material, but contained sufficient tumor cells to make a diagnosis. The tumor cells were polygonal with abundant eosinophilic cytoplasm, centrally placed nuclei and somewhat prominent nucleoli and were growing in trabecular architecture with focal pseudoacinar formation (Figure 1C). A reticulin stain showed a mildly disrupted reticulin meshwork. The tumor cells were positive for Hepar1 and arginase, along with focal glypican 3 staining. Immunostains were negative for synaptophysin, chromogranin, estrogen receptor, PAX8, and TTF-1.
Figure 1, Case 1.
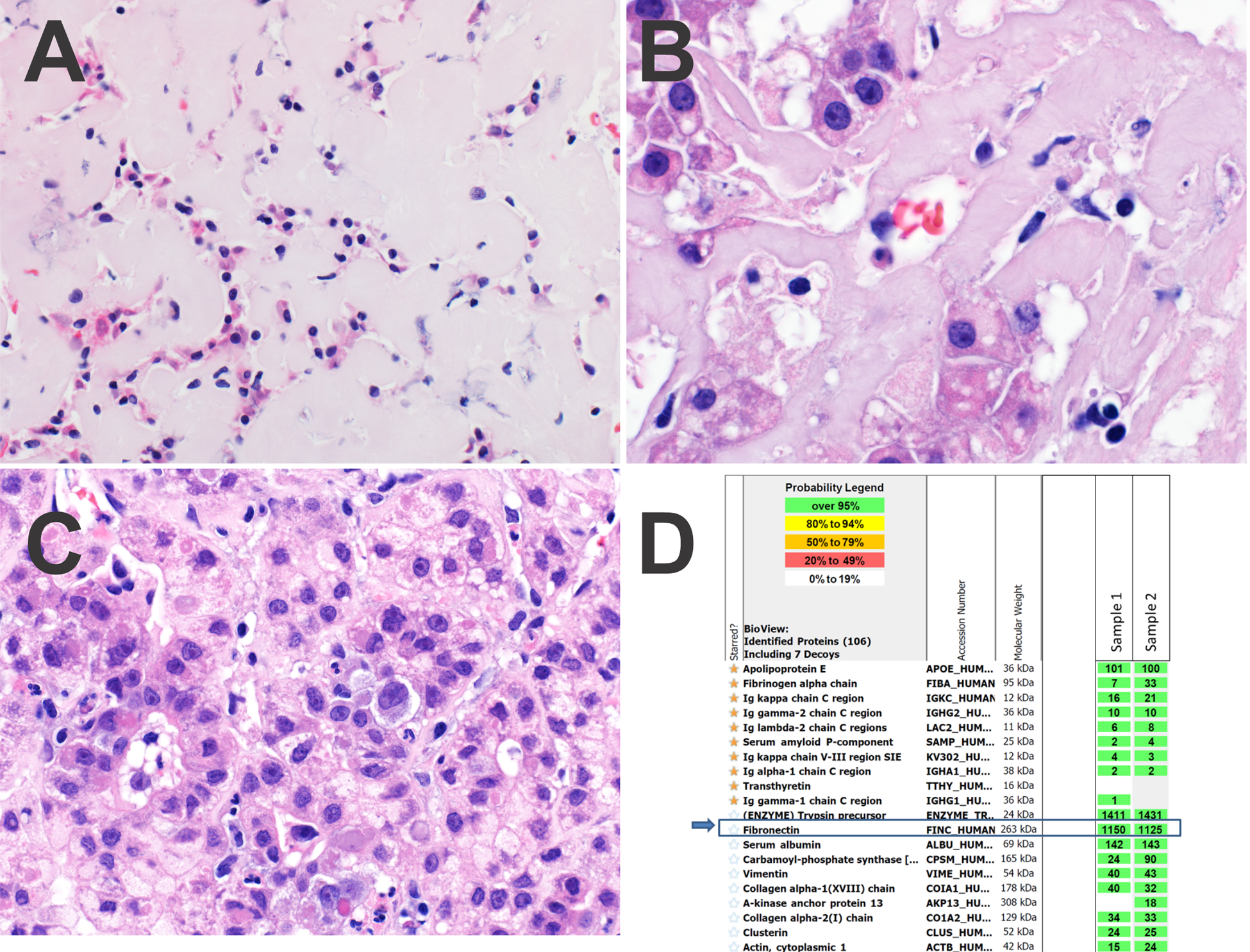
Panel A. At lower power, the cords of tumor cells are markedly compressed by extracellular deposits of fibronectin.
Panel B. At high power, the acellular fibronectin deposits have a lightly eosinophilic appearance.
Panel C. Focally, the hepatocellular carcinoma had a trabecular growth pattern with little or no fibronectin deposition.
Panel D. Protein identification reports from LC-MS/MS analysis. The extracellular deposits were microdissected in duplicate (sample 1 and 2). The starred amyloid-associated proteins are not abundant. Rather, fibronectin is the dominant protein in the deposits (blue arrows). Trypsin is present as it is the enzyme used in digestion of the peptides prior to analysis.
Additional studies were performed to identify the nature of the extracellular material. Due to its homogeneous and eosinophilic quality, amyloidosis was considered. Therefore, Congo red stains were performed, but were negative, including a repeat stain. The specimen was then sent for proteomic analysis by liquid chromatography tandem mass spectrometry (LC-MS/MS) and very high levels of fibronectin protein were identified (Figure 1D). Proteins associated with all amyloid types were not detected at levels diagnostic of amyloidosis (serum amyloid P component, apolipoprotein A IV and apolipoprotein E). Specific amyloid precursor proteins were also not identified.
Case # 2:
Clinical history
A 42-year-old man presented with chest pain and CT imaging was performed of the chest, abdomen, and pelvis, which showed a vague lesion in the dome of the liver with geographic regions of low attenuation in the left hepatic lobe with no disturbance of the underlying vasculature. The findings were thought to most likely represent fat infiltration of the liver, but a follow-up MRI was recommended. On MRI, patchy areas of relatively increased enhancement in the left hepatic lobe were noted, without precontrast T1 or T2 signal abnormalities, findings that were not suggestive of fatty infiltration but instead raised the possibility of amyloidosis versus benign perfusion anomalies. He had no significant past medical history including no history of chronic liver disease. A biopsy showed amyloid like deposits in the liver sinusoids. Systemic workup was negative for cardiovascular or renal disease. Imaging showed no masses, lymphadenopathy or organomegaly. Bone marrow analysis was negative for lymphoproliferative or plasma cell disorder. Tests for serum and urine monoclonal protein were all negative. The liver function tests were normal. He remained asymptomatic after the diagnosis.
Histologic Features
Case #2 also had 2 needle core biopsies, which showed benign, non-neoplastic liver with patchy deposition of amorphous eosinophilic, extracellular material involving approximately 10% of the biopsy cores. The extracellular deposits were accompanied by mild sinusoidal dilatation and mild atrophy of the hepatocytes cell plates and were deposited predominantly within perisinusoidal spaces (Figure 2A) and focally within portal tracts. No fibrosis was seen on trichrome stain, but the perisinusoidal amorphous deposits showed distinct pale blue staining (Figure 2B). The background liver was essentially unremarkable, without significant inflammation or fatty liver disease (Figure 2C). While there was close morphologic resemblance to amyloid, a Congo red stain was negative for congophilia and apple green birefringence. The first biopsy had limited diagnostic material, which was insufficient for confirmation of diagnosis and subtyping of the non-amyloid protein. The second biopsy was analyzed by LC-MS/MS and high levels of peptide were detected representing fibronectin (Figure 2D). Peptides associated with amyloid deposition were not identified.
Figure 2. Case 2.
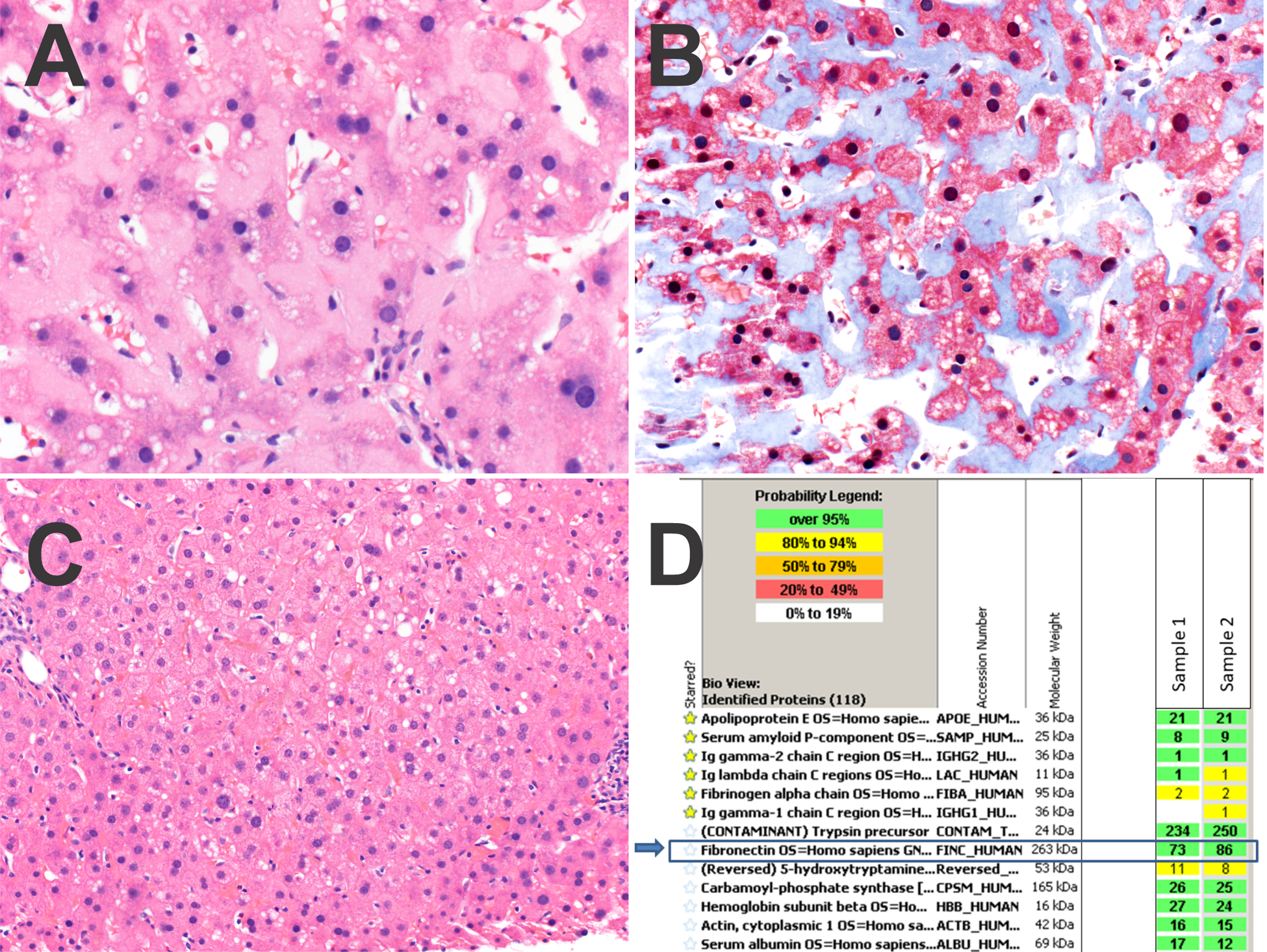
Panel A. The sinusoids show dense deposits of fibronectin.
Panel B. A trichrome stain stains the fibronectin light blue.
Panel C. The fibronectin deposition was patchy, with other areas of the biopsy negative for fibronectin.
Panel D. Protein identification reports from LC-MS/MS analysis. The extracellular deposits were microdissected in duplicate (sample 1 and 2). The starred amyloid-associated proteins are not abundant. Rather, fibronectin is the dominant protein in the deposits (blue arrows). Trypsin is present as it is the enzyme used in digestion of the peptides prior to analysis.
Discussion
We report two novel cases of fibronectin deposition in the liver, with morphological findings that closely resembled amyloidosis on H&E. In both cases, there was perisinusoidal deposition of fibronectin, sinusoidal dilatation, and atrophic hepatic cell plates. In both cases, however, Congo red stains were negative, with no congophilia and no apple green-birefringence. The differential for extracellular deposits that are amyloid like on H&E but are Congo red negative includes light chain deposition disease and Waldenstrom macroglobulinemia,4 diagnoses which can be established by clinical correlation and/or mass spectrometric analysis. Thus, in both cases the diagnostic algorithm continued on to mass spectrometric analysis, despite the negative Congo-red stains. On mass spectrometric analysis, both cases showed fibronectin deposition.
Fibronectin is produced and secreted by hepatocytes and has both a tissue and a plasma form. Under laboratory conditions, plasma fibronectin can polymerize to form beta-pleated sheets with features of amyloid.5 Also of note, similar amyloid like aggregates of fibronectin can form in tissue when there is a stressed cell microenvironment.6 In addition, amyloid like deposits of fibronectin occurs in the glomeruli of patients with fibronectin glomerulopathy, where the deposits are associated with various mutations in the FN1 gene.7 There are approximately 95 known mutations in our renal mutation database, including deletions and insertions. We were not able to identify FN gene mutations in our cases using mass spectrometry, nor could we subclassify the fibronectin as tissue fibronectin versus cellular fibronectin.
Fibronectin is a high molecular weight glycoprotein that plays an important role in cell to cell adhesion, cell to extracellular matrix adhesion, cell migration, cellular proliferation and cellular differentiation.8 Fibronectin is also important in controlling the normal growth and development of hepatocytes,9 and hepatic fibrogenesis10, 11 through complex interaction with cellular and extracellular matrix components. Fibronectin over-expression has been identified in most cases of HCC.12
It is unclear if the fibronectin deposits in case # 1 were specifically deposited in the hepatocellular carcinoma. In follow-up, we reviewed 206 resected hepatocellular carcinomas in the Mayo Clinic Rochester pathology files and found no additional cases with this morphology. Likewise, a review of 359 images of hepatocellular carcinoma included in the TCGA (https://portal.gdc.cancer.gov/) showed no additional cases. Thus, it remains unclear if the findings in case 1 represents a unique variant of hepatocellular carcinoma or more diffuse involvement of the liver that also happened to involve the well differentiated hepatocellular carcinoma, but the latter is favored. Nonetheless, tumor specific deposition cannot be excluded, since over-expression of fibronectin is well described in hepatocellular carcinoma. For example, plasma levels of fibronectin are increased in patients with hepatocellular carcinoma13, 14 and there is over-expression of protein within tumor cells.12 The mechanism for fibronectin over-expression is not clear. Somatic mutations in the FN1 gene are reported in 21 of 364 hepatocellular carcinomas in the TCGA data set (https://portal.gdc.cancer.gov/), but none of these show deposition of extracellular material on histological review.
In addition, we searched our database results of 10,654 data files submitted to mass spectrometric analysis for amyloid analysis, and found no other case of HCC with fibronectin deposition. In that process, however, a single case of fibronectin deposition in benign liver was identified, case #2. Based on these observations, the frequency of fibronectin deposition is rare both in hepatocellular carcinoma as well as in general surgical pathology specimens.
In conclusion, we report a distinctive morphological finding of extensive extracellular fibronectin deposition in a hepatocellular carcinoma as well as in non-neoplastic liver.
Grant support:
P50 CA210964 (MT)
Footnotes
Disclosures: None
References
- 1.Son RC, Chang JC, Choi JH. Primary hepatic amyloidosis: report of an unusual case presenting as a mass. Korean J Radiol. 2011;12:382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandan VS, Shah SS, Lam-Himlin DM, Petris GD, Mereuta OM, Dogan A, Torbenson MS, Wu TT. Globular hepatic amyloid is highly sensitive and specific for LECT2 amyloidosis. Am J Surg Pathol. 2015;39:558–64. [DOI] [PubMed] [Google Scholar]
- 3.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–9. [DOI] [PubMed] [Google Scholar]
- 4.Clark I, Torbenson MS. Immunohistochemistry and Special Stains in Medical Liver Pathology. Adv Anat Pathol. 2017;24:99–109. [DOI] [PubMed] [Google Scholar]
- 5.Bascetin R, Admane K, Agniel R, Boudou T, Doussineau T, Antoine R, Gallet O, Leroy-Dudal J, Vendrely C. Amyloid-like aggregates formation by blood plasma fibronectin. Int J Biol Macromol. 2017;97:733–743. [DOI] [PubMed] [Google Scholar]
- 6.Bascetin R, Blay L, Kellouche S, Carreiras F, Picot CR, Briand M, Agniel R, Gallet O, Vendrely C, Leroy-Dudal J. Fibronectin amyloid-like aggregation alters its extracellular matrix incorporation and promotes a single and sparsed cell migration. Exp Cell Res. 2018;371:104–121. [DOI] [PubMed] [Google Scholar]
- 7.Castelletti F, Donadelli R, Banterla F, Hildebrandt F, Zipfel PF, Bresin E, Otto E, Skerka C, Renieri A, Todeschini M, Caprioli J, Caruso RM, Artuso R, Remuzzi G, Noris M. Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc Natl Acad Sci U S A. 2008;105:2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K Fibronectin and other cell interactive glycoproteins, In: Hay ED, (ed). Cell biology of extracellular matrix. Plenum Press: New York; 1989. pp 111–46. [Google Scholar]
- 9.Kosmehl H, Berndt A, Katenkamp D. Molecular variants of fibronectin and laminin: structure, physiological occurrence and histopathological aspects. Virchows Arch. 1996;429:311–22. [DOI] [PubMed] [Google Scholar]
- 10.Odenthal M, Neubauer K, Meyer zum Buschenfelde KH, Ramadori G. Localization and mRNA steady-state level of cellular fibronectin in rat liver undergoing a CCl4-induced acute damage or fibrosis. Biochim Biophys Acta. 1993;1181:266–72. [DOI] [PubMed] [Google Scholar]
- 11.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torbenson M, Wang J, Choti M, Ashfaq R, Maitra A, Wilentz RE, Boitnott J. Hepatocellular carcinomas show abnormal expression of fibronectin protein. Mod Pathol. 2002;15:826–30. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Park J, Kim Y, Sohn A, Yeo I, Jong Yu S, Yoon JH, Park T, Kim Y. Serum fibronectin distinguishes the early stages of hepatocellular carcinoma. Sci Rep. 2017;7:9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attallah AM, El-Far M, Abdel Malak CA, Farid K, Omran MM, Yahya RS, Saad EA, Albannan MS, Attallah AA, El Basuni MA, Ali IS, Abed SB, El Naggar MA. A simple diagnostic index comprising epithelial membrane antigen and fibronectin for hepatocellular carcinoma. Ann Hepatol. 2015;14:869–80. [DOI] [PubMed] [Google Scholar]


