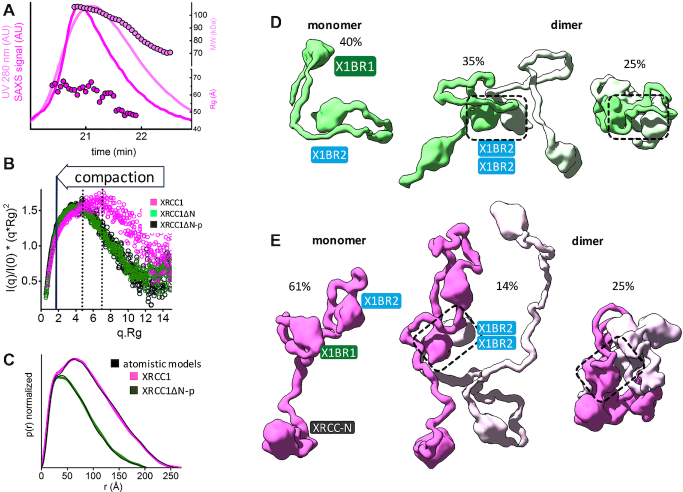
Figure 2.
Human XRCC1 is an elongated, disordered protein that transiently forms homodimers. (A) SEC-SAXS-MALS chromatographs for XRCC1. Solid lines represent the UV 280 nm (light magenta) or SAXS signal (magenta) in arbitrary units, while symbols represent molecular mass (light magenta) and Rg values for each collected SAXS frame (magenta) versus elution time. The SEC-SAXS-MALS results, which show full-length XRCC1 is a mixture of monomer/dimer, are representative of at least two independent preparations of XRCC1. (B) Normalized Kratky plot of XRCC1 (magenta) in comparison with XRCC1ΔN-p (dark green) and XRCC1ΔN (light green). (C) Normalized P(r) functions of XRCC1 (magenta) in comparison with XRCC1ΔN-p (dark green) matching theoretical P(r) functions of atomistic models (black) shown in panels D and E. Weighted ensemble atomistic model shown in molecular surface representation were used to fit experimental SAXS curves for XRCC1ΔN-p (D) and XRCC1 (E) shown in Supplementary Figure S7A and represented as theoretical P(r) functions in panel C.