Figure 3.
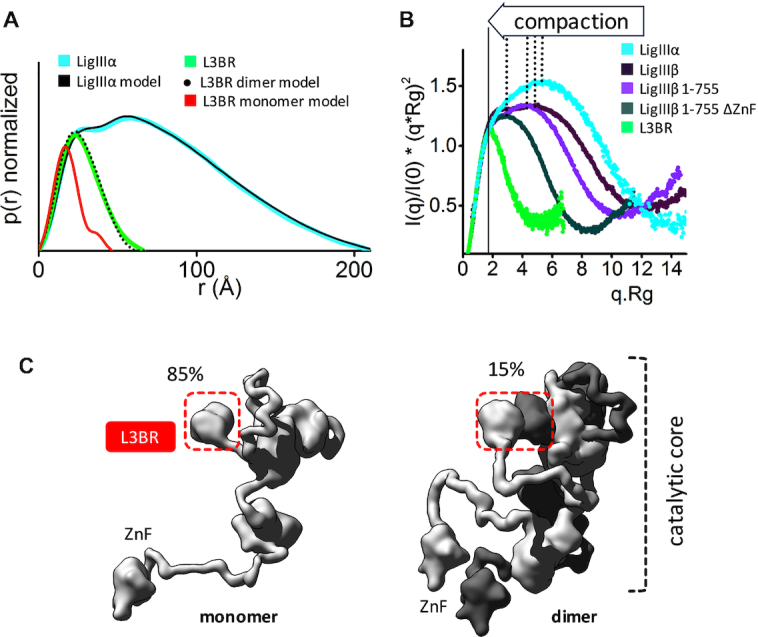
Human LigIIIα is an elongated, disordered protein that transiently forms homodimers. (A) Normalized P(r) functions for experimental SAXS curves for full length LigIIIα (cyan) and L3BR (green) are fitted to the theoretical P(r) functions (black) of atomistic models of full length LigIIIα (panel C) and the two-fold symmetric crystal structure of the L3BR homodimer (PDBID: 3PC7, black dots) and L3BR monomer (red). (B) Comparison of normalized Kratky plots of LigIIIα (cyan), L3BR dimer (green) with the SAXS curves of LigIIIβ (dark violet), LigIIIβ 1–755 (violet), and LigIIIβ 1–755 ΔZnF (dark green) (taken from (63)). Kratky plots show persistent disorder of full length LigIIIα and LigIIIβ but significant less disorder in the LigIIIβ construct with truncated ZnF domain. (C) Weighted ensemble atomistic models of LigIIIα monomer (left) and homodimer (right) are shown in surface representation. Corresponding fits of the SAXS curves for the atomistic models are shown in the Supplementary Figure S7D and as a theoretical P(r) function in panel A.
