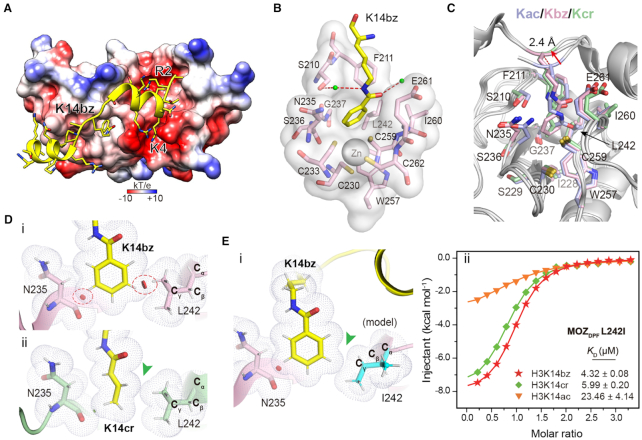
Figure 2.
Molecular details for histone H3K14bz recognition by MOZDPF. (A) Overall structure of MOZDPF in complex with H31–25K14bz. MOZDPF is shown as electrostatic potential surface ranging from −10 (red) to +10 (blue) kT/e. Histone H3 is shown as yellow cartoon with residues depicted as sticks. (B) Recognition of K14bz mark by the hydrophobic reader pocket of MOZDPF. Small green balls, water molecules; Red dashes, hydrogen bonds; Gray sphere, zinc ion. (C) Superimposition of K14bz-bound (pink), K14ac-bound (light blue, PDB: 4LLB) and K14cr-bound (pale green, PDB ID: 5B76) MOZDPF structures. Key pocket residues are depicted as sticks; Red arrow highlights the up-lift of the H3K14bz backbone. (D) Close contact analyses of MOZDPF structure bound to (i) H3K14bz and (ii) H3K14cr (PDB ID: 5B76). Gray dots denote van der Waals surface of the indicated residues. Large red disk, severe van der Waals overlap; Small green disk, slight van der Waals overlap. Red circles and green arrowhead highlight steric clashes in H3K14bz imbedded structure and spatial compatibility in H3K14cr imbedded structure, respectively. Hydrogens (white sticks) were added for analysis. (E) i, structural modeling of L242I mutation of MOZDPF bound to H3K14bz. Note the loss of ‘close contact’ in L242I mutant (green arrowhead); ii, ITC fitting curves of the indicated histone peptides with L242I mutant. Mean KD and standard deviation are shown (N = 2).