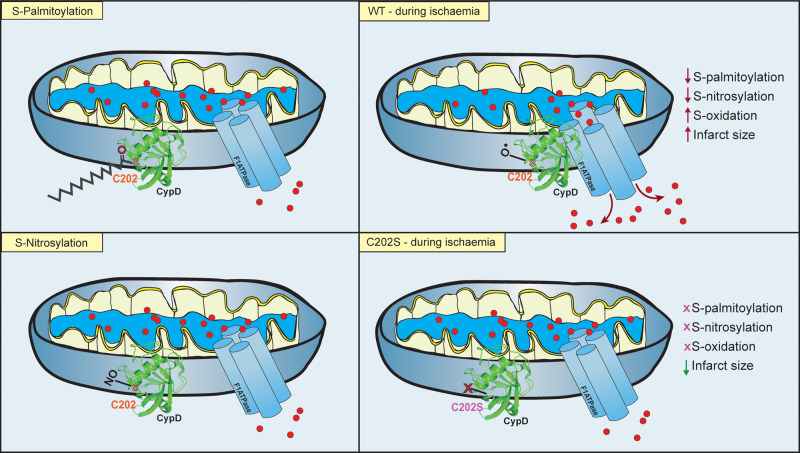
Figure 1.
CypD C202 Modification in Cardiac Mitochondria. Amanakis et al. demonstrate that under basal conditions, the mitochondrial permeability transition pore (PTP) regulator cyclophillin-D (CypD) is reported to be both S-nitrosylated and S-palmitoylated at position cysteine 202 (C202). Under conditions of ischaemia, CypD S-palmitoylation and S-nitrosylation are reduced as C202 undergoes oxidation. This leads to greater association of CypD with the F1-ATPase subunit of the PTP and enhances pore opening and calcium efflux at reperfusion, thus leading to greater myocardial damage and increased infarct size. Mutation of C202 to a serine (S) results in no possible modification by S-nitrosylation, S-palmitoylation but also S-oxidation and transgenic mice have significantly reduced infarct size and enhanced functional recovery as a result.