Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a rare, but serious systemic hypersensitivity reaction associated with a range of medications. We present two cases of vancomycin-induced DRESS, which occurred simultaneously in the orthopaedic ward in an outer metropolitan hospital. These cases demonstrate the complexity in the diagnosis and management of this inflammatory syndrome on the background of known infection as well as evidence for linezolid as an alternative to vancomycin. The first case was managed conservatively, but developed progressive renal and liver injury along with demonstrated cytomegalovirus reactivation and recurrent colitis, and was eventually palliated. The second was commenced on intravenous glucocorticoids and achieved remission, although had ongoing renal dysfunction at the time of discharge from outpatient follow-up.
Keywords: dermatology, drugs and medicines, unwanted effects / adverse reactions, bone and joint infections, immunology
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) is classified among the severe cutaneous adverse drug reactions (SCARs). It typically presents with a skin eruption, fever, eosinophilia and internal organ involvement.1 Diagnosis requires identification of a systemic delayed response from a likely associated exposure (typically 2–8 weeks); the RegiSCAR scoring system2 is one of the most widely used clinical decision tools for this purpose.
Vancomycin-induced DRESS is a rare entity. Classically, anticonvulsants and allopurinol have been considered to be the most common causative agents of DRESS,1 3 although rates between studies and genetically distinct populations vary. A systematic review by Minhas et al4 found a total of 16 cases of DRESS between 1982 and 2015, while Lam and colleagues5 reported 12 within 3 years in their institution. In the second study, antibiotics were found to be the causative agent in 63% of cases, of which 60% were associated with vancomycin.
Predisposition to DRESS involves an interplay between immunogenetics (in particular HLA-A*32:01 for vancomycin6) and underlying drug metabolism, with CYP enzyme polymorphisms, age and renal function, all associated with risk.7–10 In the future, HLA typing may be useful not just for confirming diagnosis and identifying candidate drug causes,6 but also predicting cross-reactivity to other drugs, such as vancomycin and other glycopeptides,11 although this is not yet regular clinical practice. The underlying pathophysiological mechanism for DRESS remains to be fully elucidated, although the syndrome is notable for a dysregulated T-cell response as well as serial reactivation of herpesviruses, which may be involved in the prolonged duration or relapse of the disease.7–12
Management of DRESS requires prompt recognition and cessation of all possible causative agents. Immunosuppression is often added, given the mortality risk. The current gold standard approach to immunosuppression uses prednis(ol)one13 while success has also been reported with cyclosporine,14 tumour necrosis factor inhibitors15 and mepolizumab.16 The addition of immunosuppression is particularly complex where the causative drug is an antibiotic and there is suspicion of ongoing active infection. Even with active management, mortality may occur in up to 10% of cases.1
Case presentation—one
A 70-year-old Caucasian woman with a background of ischaemic heart disease and chronic obstructive pulmonary disease presented electively on 7 November 2018 for the second-stage revision of an infected left total hip replacement, having received a 6-week course of intravenous vancomycin in 2016 as directed therapy for Staphylococcus epidermidis. This original course was well tolerated although a transient eosinophilia of 0.71×109/L (reference <0.4×109/L) above a baseline of 0.3×109/L was noted.
Her preadmission medications included a recently completed course of amoxicillin for exacerbation of airways disease and long-term medications of aspirin, ramipril, rosuvastatin, pregabalin, pantoprazole, paracetamol, and inhaled budesonide/formoterol and terbutaline.
Remnant necrotic tissue was noted intraoperatively, and deep tissue specimens again cultured vancomycin-sensitive S. epidermidis. Vancomycin was restarted on 14 November 2018 and she was discharged from the hospital with ongoing vancomycin via continuous elastometric infusors.
She re-presented to the emergency department on 2 December 2018 (day 19 of vancomycin) with fever of 39°C, heart rate of 110/min, and a widespread subtle pink rash on the limbs, trunk and neck. CT scanning of the pelvis demonstrated a 59×33 mm periprosthetic collection with associated fat stranding and iliac and inguinal lymphadenopathy. A preliminary diagnosis of uncontrolled joint infection with a superimposed heat rash was made, with a plan for repeat debridement.
Repeat washouts were performed on 3 and 5 December 2018, followed by a final washout and closure on the 12 December 2018, which included the addition of vancomycin-loaded cement. Intraoperative tissue samples demonstrated active inflammation with the presence of eosinophils.
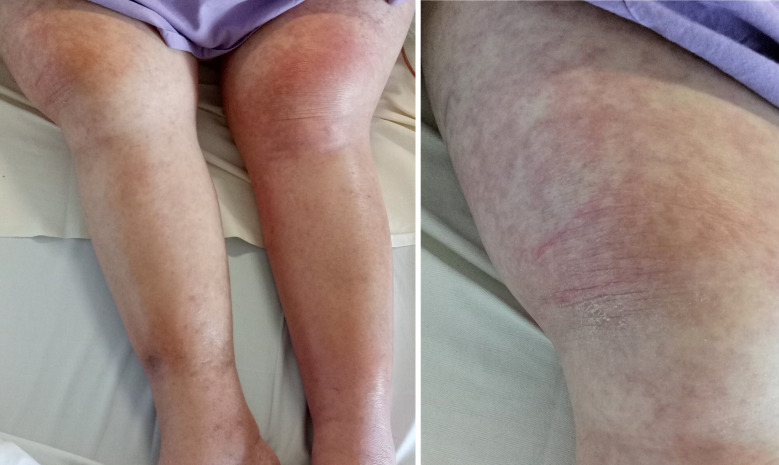
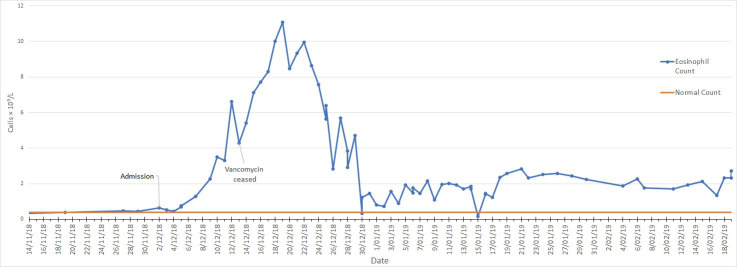
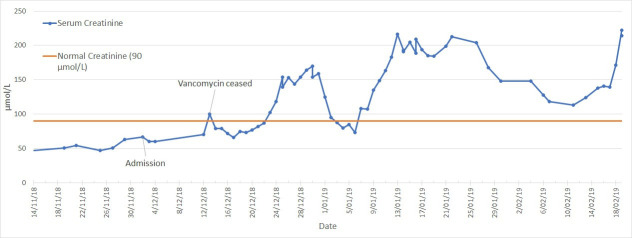
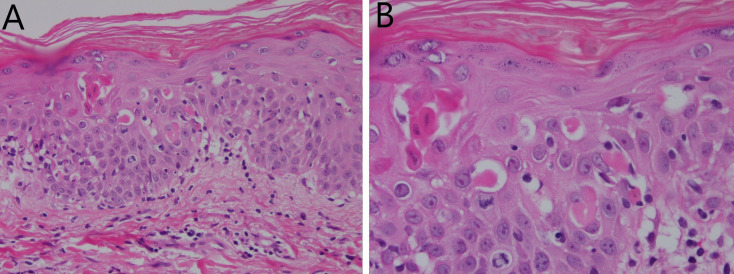
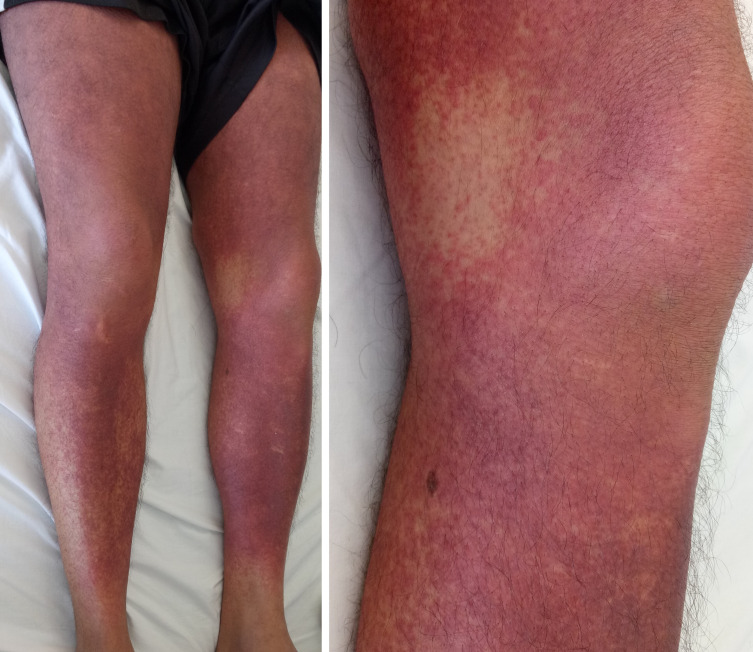
Despite extensive attempts at source control, the patient had persistent fevers and rash (figure 1). A rising eosinophil count (figure 2) was noted, which was subsequently followed by deteriorating renal function (from a normal baseline) and liver enzyme derangement (figures 3 and 4). Normal ranges are based on those used by Royal College of Pathologists of Australasia.17 18 A diagnosis of probable DRESS was made based on a RegiSCAR score of 5, which was confirmed by punch biopsy (figure 5).
Figure 1.
Rash on lower limbs of patient one.
Figure 2.
Eosinophil count from vancomycin initiation until palliation in patient one with vancomycin cessation on 13 December 2018.
Figure 3.
Serum creatinine temporal trend in patient one. There was a progressive rise in serum creatinine despite drug cessation on 13 December 2018.
Figure 4.
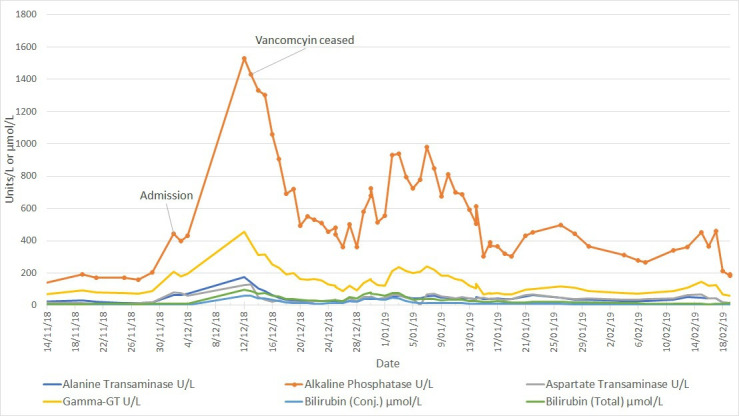
Liver enzyme temporal trend in patient one showing partial resolution following vancomycin cessation on 13 December 2018. Testing dates marked on alkaline phosphatase trend.
Figure 5.
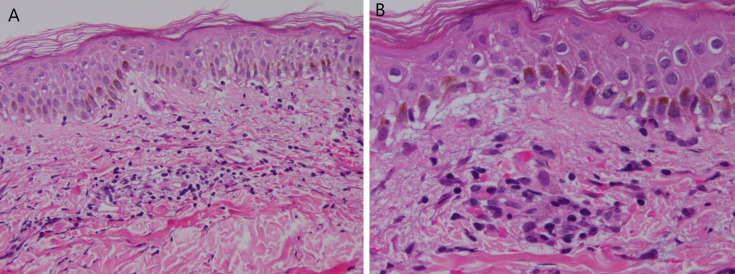
Skin biopsy (H&E stain) described as erythema multiforme-like lichenoid reaction pattern consistent with drug reaction at ×200 (A) and ×400 (B) magnification.
Treatment
As the only new drug exposure, vancomycin was rationalised to linezolid on 13 December 2018, avoiding a risk of cross-reactivity with teicoplanin. Glucocorticoids and other immunosuppressants were avoided due to underlying frailty and surgical concern about persistent infection. Linezolid was transitioned to pristinamycin for maintenance therapy on 25 January 2019.
The role of intra-articular vancomycin-loaded cement has previously been explored in the literature19 20 and is thought to have limited impact on disease prognosis. Further surgical intervention was, therefore, not considered appropriate.
Outcome and follow-up
Despite drug withdrawal, there was evidence of progressive renal failure (figure 3), although with associated improvement in haematologic and hepatic markers (figures 2 and 4). The eosinophil count showed partial response, peaking at 11.08×109/L on 19 December 2018 before declining to range between 1 and 3×109/L.
Significant gastrointestinal inflammation developed with persistent diarrhoea and a stool calprotectin of 77 μg/g recorded on 17 December 2018 (reference <50 µg/g). PCR testing for infectious causes was performed, with four stool samples returning negative results for common viral, bacterial and protozoal pathogens between 17 December 2018 and 5 February 2019. Clostridioides screening was also performed on five stool samples between 13 December 2018 and 5 February 2019, all of which returned negative results.
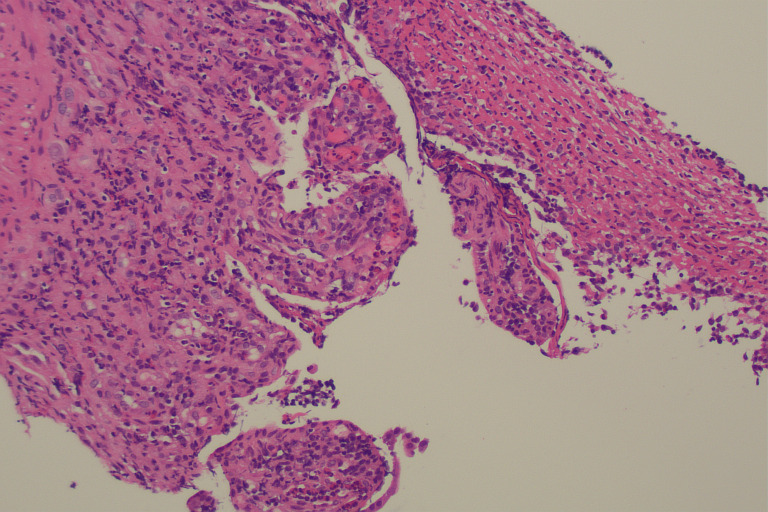
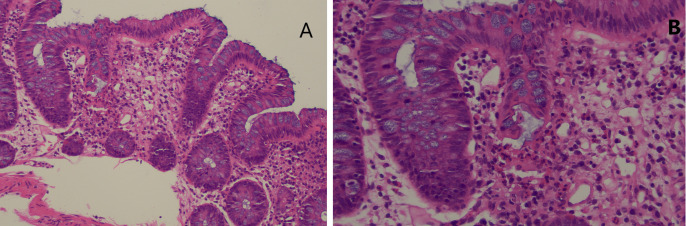
Due to rising faecal calprotectin levels (710 μg/g on 20 December 2018) and ongoing diarrhoea, a colonoscopy was performed on 20 December 2018. Histology of the terminal ileum and colon (figures 6 and 7) demonstrated a mixed inflammatory infiltrate with active ileitis and ulceration, while the colonic samples showed mild active colitis with cryptitis but no evidence of erosion or ulceration. These findings were considered to be non-specific, although suggestive of a resolving infectious or drug-induced colitis.
Figure 6.
Terminal Ileal biopsy demonstrating ulceration with acute inflammatory change (H&E stain ×100 magnification).
Figure 7.
Colonic biopsy (H&E stain) demonstrating patchy cryptitis within normal mucosa (A, ×100 magnification) and crypt rupture (B, ×200 magnification).
No viral cytopathic changes were noted on histological examination and PCR testing for cytomegalovirus (CMV) DNA on the tissue specimen was negative. CMV serology done on 15 December 2018 showed reactivity to IgG, but not IgM, indicating past exposure with latent infection.
No change to management was made, and faecal calprotectin levels fell to 280 μg/g on 2 January 2019. Seroconversion of CMV was detected with IgM reactivity on 6 January 2019 and was followed by further gastrointestinal inflammation. An episode of melaena led to upper gastrointestinal endoscopy on 15 January 2019, which demonstrated a gastric ulcer. This was managed with adrenaline and bipolar cautery without further bleeding.
Given progressive renal and gastrointestinal failure, patient one was palliated on 20 January 2019 and further invasive investigation was considered contraindicated. Relapsed colitis was diagnosed by faecal calprotectin rising to 1200 μg/g on 23 January 2019, which was thought likely either a rare manifestation of DRESS or possible CMV colitis given seroconversion. Repeat colonoscopy was avoided given her palliative status.
Patient one passed away on 21 February 2019 in the palliative care unit of the hospital.
Case presentation—two
A 46-year-old Indian man presented to hospital on 23 October 2018 with infected non-union of a fractured left fifth phalanx, initially conservatively managed in India following a motorcycle accident 4 weeks prior. He was taking no regular medications at the time of admission. Surgical washout and debridement was performed on 30 October 2018 and intravenous cefazolin commenced empirically. Intraoperative tissue specimens cultured multiresistant S. aureus and antimicrobial therapy was changed to vancomycin on 2 November 2018, with a plan for 6 weeks of treatment from the date of the final operation. A planned repeat washout was performed on 4 December 2018 demonstrating osteomyelitis.
A generalised erythematous rash (figure 8) was first noted on 6 December 2018 (day 35 of vancomycin), in association with fever and acute liver and kidney injury. This was initially observed, but the rash did not improve.
Figure 8.
Rash on lower limbs of patient two.
On 13 December 2018, the diagnosis of DRESS was made based on a RegiSCAR score of 7 indicating definite DRESS, which was further supported by punch biopsy (figure 9) on 14 December 2018. This was prompted by the earlier diagnosis of patient one, who was in the same ward.
Figure 9.
Skin biopsy reported as epidermal spongiosis and superficial dermal perivascular inflammation (H&E stain) at ×200 (A) and ×400 (B) magnification. This pattern is considered to be non-specific but commonly seen in drug reaction with eosinophilia and systemic symptoms.
Nephrotic range proteinuria was detected on 14 December 2018 with a random protein:creatinine ratio of 21 g/mol of creatinine. Progression of eosinophilia is noted in figure 10, and hepatic and renal function trends are shown in figure 11.
Figure 10.
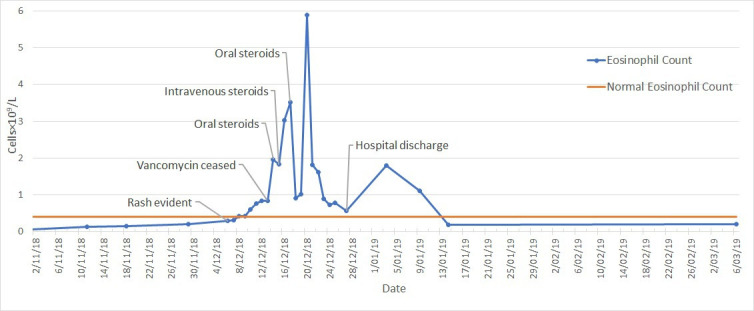
Eosinophil count from vancomycin initiation on 2 November 2018 until end of outpatient follow-up in patient two.
Figure 11.
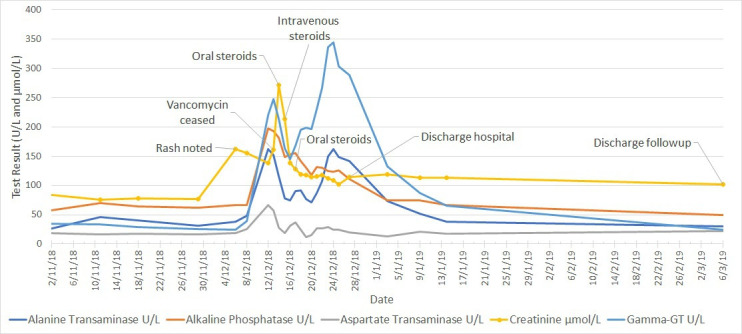
Liver enzyme and serum creatinine temporal trend in patient two, showing persisting kidney injury at discharge from outpatient follow-up on 6 March 2019. There was no significant elevation in bilirubin levels in this case.
Treatment
On 13 December 2018, vancomycin was ceased and linezolid commenced. As with case one, teicoplanin was avoided.
Despite drug cessation, the patient’s renal function and widespread skin rash continued to worsen. Given his lower risk of infection and better performance status, high-dose oral prednisolone (0.5 mg/kg) was initiated on 14 December 2018. This was escalated to 1.25 mg/kg after worsening symptoms and review of biochemistry.
No further surgical intervention was required.
Outcome and follow-up
The rash and renal function improved with steroid therapy and he commenced a tapering course of prednisone on 17 December 2018. On 27 December 2018, patient two was discharged from hospital on 30 mg of prednisolone, 600 mg two times per day of oral linezolid and prophylactic trimethoprim/sulfamethoxazole 160/800 mg per day.
Normalisation of liver function occurred by 6 March 2019, showing a significant lag behind clinical improvement, although there was ongoing evidence of renal dysfunction with a creatinine of 102 (baseline 70–85) on that date.
Patient two was discharged from specialist follow-up on 6 March 2019. No further engagement with our service has been required as of June 2020.
Discussion
We presented two cases of vancomycin-induced DRESS in concurrent orthopaedic inpatients. Both cases showed core features of the syndrome with the occurrence of fevers, rash and visceral involvement2 after prolonged durations of therapy.21 Vancomycin-induced DRESS appears to be particularly associated with renal function impairment,13 as was demonstrated in both cases, although this is also a common manifestation of DRESS. Prednisolone and drug cessation was effective in inducing remission in patient two, while cessation alone was ineffective in patient one, leading to progressive multiorgan failure and death.
Notable in patient one was the development of significant gastrointestinal inflammation, which is rarely reported as a manifestation in DRESS; the RegiSCAR prospective study2 reported only 6 cases of gastrointestinal involvement from their total 117 definite and probable cases. Our case also appears to be the first published evidence of this in association with vancomycin. We theorise that this represented either a primary DRESS manifestation or a superimposed CMV colitis given the subsequent seroconversion; however, it was not possible to confirm this given her clinical deterioration.
We recommend practitioners consider colonoscopy and microbiological testing in patients with DRESS and gastrointestinal symptoms or signs to further define the aetiology and possible treatment direction for this manifestation.
Management of DRESS, in general, remains controversial. While glucocorticoids are considered gold standard22 and were used with success in our second case, concerns around patient’s vulnerability to infection led to a supportive approach in case one, which has also been described in the literature7 as an appropriate alternative. Both cases demonstrated linezolid as a viable alternative for vancomycin where infectious concerns persist.
Given the rarity of DRESS, the detection of two simultaneous cases in a peripheral hospital raised drug safety concerns. Batch contamination was excluded by liaison with infectious diseases, antimicrobial stewardship, and medication safety pharmacist networks, both statewide and nationwide, as well as the Therapeutic Goods Administration (TGA), the Australian national drug regulation and licensing body. No further cases have been reported in association with the vancomycin batches used for patients one and two, or with any other batches used at our institution, as of June 2020. Additionally, no epidemiological links were identified by the TGA.
Learning points.
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a rare and potentially fatal complication of vancomycin therapy, particularly with prolonged courses.
The diagnosis should be suspected in patients with eosinophilia, rash and fevers, avoiding the assumption that continued fever represents ongoing infection.
Gastrointestinal inflammation can complicate the clinical course and should be considered in the diagnosis, management, and research of DRESS.
Glucocorticoids can be considered for induction of remission of DRESS while continuing therapy for orthopaedic infections with an alternative antimicrobial agent.
Acknowledgments
Dr John Pauli, SMO, Anatomical Pathologist, Pathology Queensland, The Prince Charles Hospital, 627 Rode Rd, Chermside, Queensland 4032, for providing histopathological images and interpretation, particularly in relation to manifestations of drug reaction with eosinophilia and systemic symptoms.
Footnotes
Contributors: MC diagnosed drug reaction with eosinophilia and systemic symptoms in case 1 which led to the subsequent diagnosis of case 2 by the remaining authors. Direct patient care for both cases on an ongoing basis was provided by SP, SG and RC. SG undertook chart reviews for both cases during article production. All authors were involved in the background research and writing of the final article with supervision by RC.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Husain Z, Reddy BY, Schwartz RA. Dress syndrome: Part I. clinical perspectives. J Am Acad Dermatol 2013;68:693.e1. 10.1016/j.jaad.2013.01.033 [DOI] [PubMed] [Google Scholar]
- 2.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. . Drug reaction with eosinophilia and systemic symptoms (dress): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol 2013;169:1071–80. 10.1111/bjd.12501 [DOI] [PubMed] [Google Scholar]
- 3.Peter JG, Lehloenya R, Dlamini S, et al. . Severe delayed cutaneous and systemic reactions to drugs: a global perspective on the science and art of current practice. J Allergy Clin Immunol Pract 2017;5:547–63. 10.1016/j.jaip.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minhas JS, Wickner PG, Long AA, et al. . Immune-Mediated reactions to vancomycin: a systematic case review and analysis. Ann Allergy Asthma Immunol 2016;116:544–53. 10.1016/j.anai.2016.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam BD, Miller MM, Sutton AV, et al. . Vancomycin and dress: a retrospective chart review of 32 cases in Los Angeles, California. J Am Acad Dermatol 2017;77:973–5. 10.1016/j.jaad.2017.05.041 [DOI] [PubMed] [Google Scholar]
- 6.Konvinse KC, Trubiano JA, Pavlos R, et al. . HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol 2019;144:183–92. 10.1016/j.jaci.2019.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho Y-T, Yang C-W, Chu C-Y. Drug reaction with eosinophilia and systemic symptoms (dress): an interplay among drugs, viruses, and immune system. Int J Mol Sci 2017;18. 10.3390/ijms18061243. [Epub ahead of print: 09 Jun 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung W-H, Chang W-C, Lee Y-S, et al. . Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA 2014;312:525–34. 10.1001/jama.2014.7859 [DOI] [PubMed] [Google Scholar]
- 9.Hung S-I, Chung W-H, Liou L-B, et al. . HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005;102:4134–9. 10.1073/pnas.0409500102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Génin E, Schumacher M, Roujeau J-C, et al. . Genome-Wide association study of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe. Orphanet J Rare Dis 2011;6:52. 10.1186/1750-1172-6-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakkam N, Gibson A, Mouhtouris E, et al. . Cross-reactivity between vancomycin, teicoplanin, and telavancin in patients with HLA-A∗32:01-positive vancomycin-induced DRESS sharing an HLA class II haplotype. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.04.056. [Epub ahead of print: 19 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y-C, Chang C-Y, Cho Y-T, et al. . Long-Term sequelae of drug reaction with eosinophilia and systemic symptoms: a retrospective cohort study from Taiwan. J Am Acad Dermatol 2013;68:459–65. 10.1016/j.jaad.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 13.Madigan LM, Fox LP. Vancomycin-associated drug-induced hypersensitivity syndrome. J Am Acad Dermatol 2019;81:123–8. 10.1016/j.jaad.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 14.Zuliani E, Zwahlen H, Gilliet F, et al. . Vancomycin-induced hypersensitivity reaction with acute renal failure: resolution following cyclosporine treatment. Clin Nephrol 2005;64:155–8. 10.5414/CNP64155 [DOI] [PubMed] [Google Scholar]
- 15.Leman RE, Chen L, Shi X, et al. . Drug reaction with eosinophilia and systemic symptoms (dress) successfully treated with tumor necrosis factor-α inhibitor. JAAD Case Rep 2017;3:332–5. 10.1016/j.jdcr.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ange N, Alley S, Fernando SL, et al. . Drug reaction with eosinophilia and systemic symptoms (dress) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract 2018;6:1059–60. 10.1016/j.jaip.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 17.Royal College of Pathologists Australia White cell count differential. secondary white cell count differential, 2019. Available: https://www.rcpa.edu.au/Manuals/RCPA-Manual/Pathology-Tests/W/White-cell-count-differential
- 18.Royal College of Pathologists Australia Creatinine. Secondary creatinine, 2019. Available: https://www.rcpa.edu.au/Manuals/RCPA-Manual/Pathology-Tests/C/Creatinine
- 19.Güner MD, Tuncbilek S, Akan B, et al. . Two cases with HSS/DRESS syndrome developing after prosthetic joint surgery: does vancomycin-laden bone cement play a role in this syndrome? BMJ Case Rep 2015;2015. 10.1136/bcr-2014-207028. [Epub ahead of print: 28 May 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper KD, Incavo SJ. Drug reaction with eosinophilia and systemic symptoms syndrome after total knee arthroplasty infection and placement of antibiotic spacer. Arthroplast Today 2019;5:148–51. 10.1016/j.artd.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumenthal KG, Peter JG, Trubiano JA, et al. . Antibiotic allergy. Lancet 2019;393:183–98. 10.1016/S0140-6736(18)32218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiohara T, Kano Y. Drug reaction with eosinophilia and systemic symptoms (dress): incidence, pathogenesis and management. Expert Opin Drug Saf 2017;16:139–47. 10.1080/14740338.2017.1270940 [DOI] [PubMed] [Google Scholar]