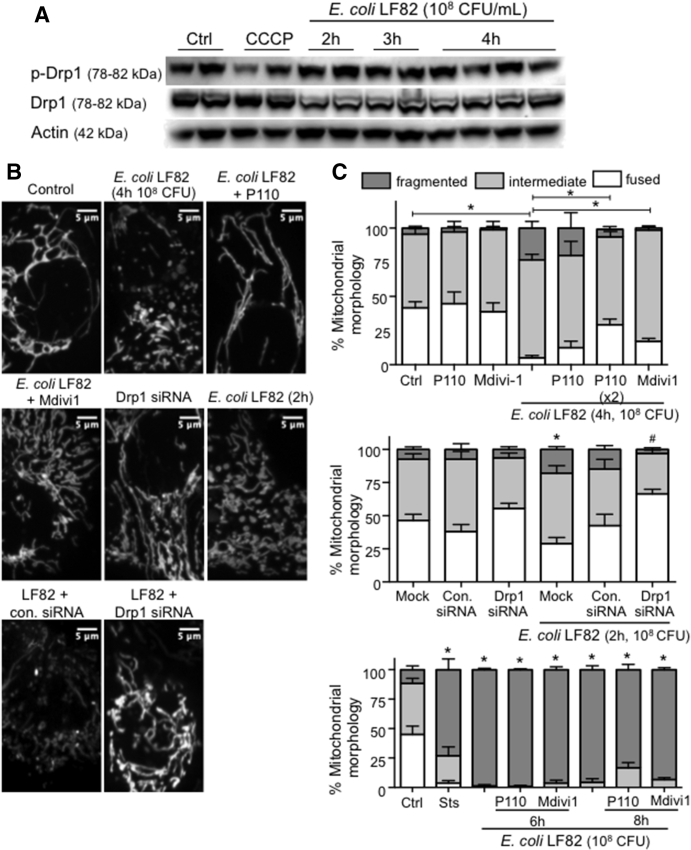
Figure 6.
Inhibition of Drp1 activity reduces mitochondrial fragmentation induced by adherent-invasive E coli–LF82. (A) Western blot and densitometry analysis showing that neither total nor phosphorylated Drp1 (Ser616 site) protein expression in whole-cell lysates is altered consistently in T84 epithelia infected with E coli–LF82. (B and C) Inhibition of Drp1 activity with P110 (10 μmol/L) or Mdivi1 (5 μmol/L) or siRNA (30 nmol/L) knockdown of Drp1 protein reduces the severity of E coli–LF82–evoked T84 epithelial cell mitochondrial fragmentation assessed at 2 and 4 hours after infection, but not at 6 or 8 hours after exposure to the viable bacteria. Representative images of MitoTracker-stained mitochondrial networks. Middle: ∗P < .05 compared with mock transfection uninfected; #P < .05 compared with mock transfection infected. Bottom: ∗P < .05 compared with control (ctrl), 2-way analysis of variance followed by the Tukey multiple comparison test; n = 4–11 epithelial monolayers (20 cells/monolayer), 2–4 experiments. con.siRNA, control siRNA; Sts, 20 μmol/L staurosporine. Data are means ± SEM.