Fig. 5.
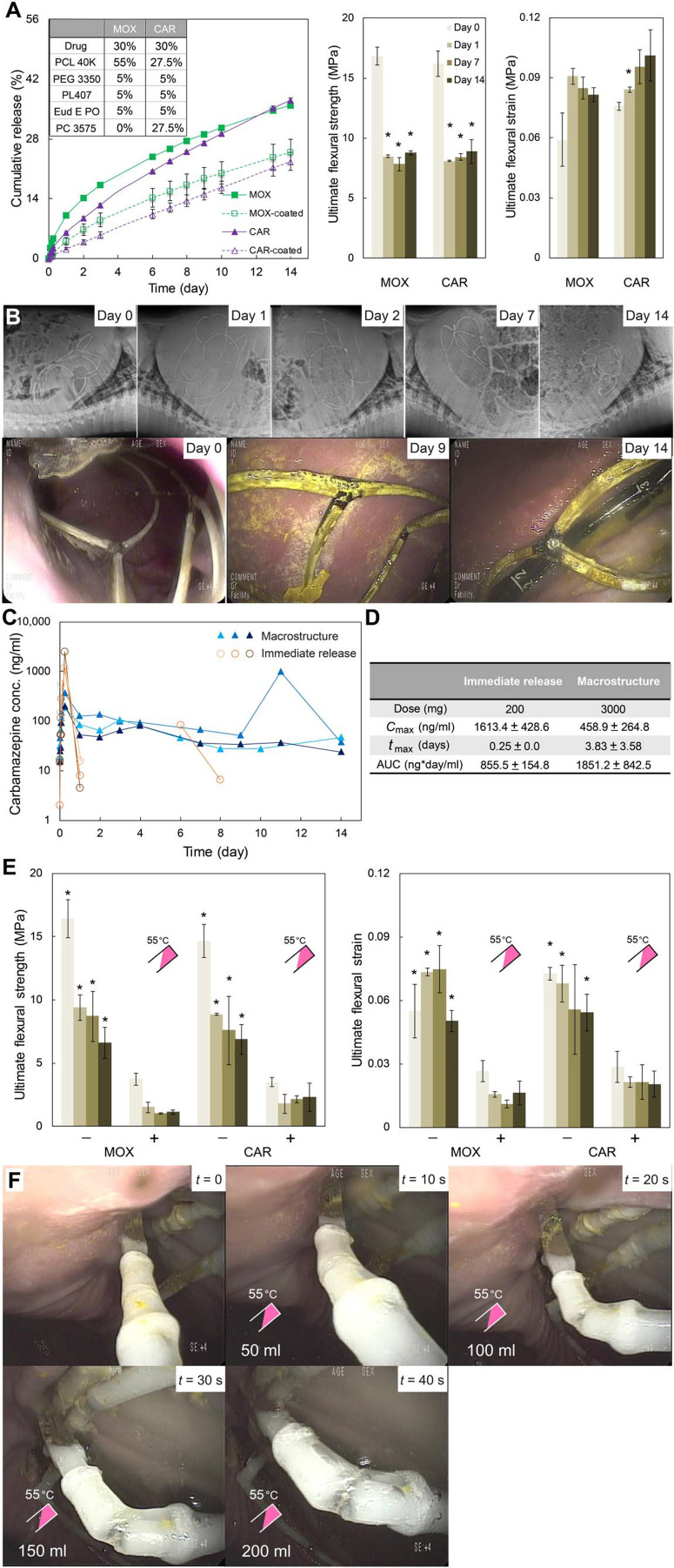
Characterization of metamaterial dosage formin vitro and in vivo. (A) In vitro cumulative drug release and ultimate flexural strength and strainof drug-loaded arms for MOX and CAR formulations incubated in 37°C SGFfor 14 days. Markers and column bars represent the mean ± SD for n = 3samples per group. *P < 0.05, one-way ANOVA and post hoc Bonferronimultiple comparison tests were used to determine the significance (days 1, 7, and 14 versus day 0). (B) Representative abdominal radiographs and endoscopic images obtained at various time points after metamaterialdosage form administration. (C) Serum concentration profiles over 15 days and (D) pharmacokinetic analysis of CAR administered as an immediaterelease formulation (orange) versus macrostructure dosage form (blue). (E) Effect of temperature on ultimate flexural strength and strain ofdrug-loaded arm segments with TRLs incubated in 37°C SGF for 14 days. Two-sample t tests were used to determine the significance. *P < 0.05 [not exposed to warm water (−) versus exposed to warm water (+)]. (F) In vivo endoscopic images showing the weakening sequence of a TRL for a macrostructureafter spraying 200 ml of 55°C water 7 days after gastric metamaterialdosage form deployment in a porcine model.