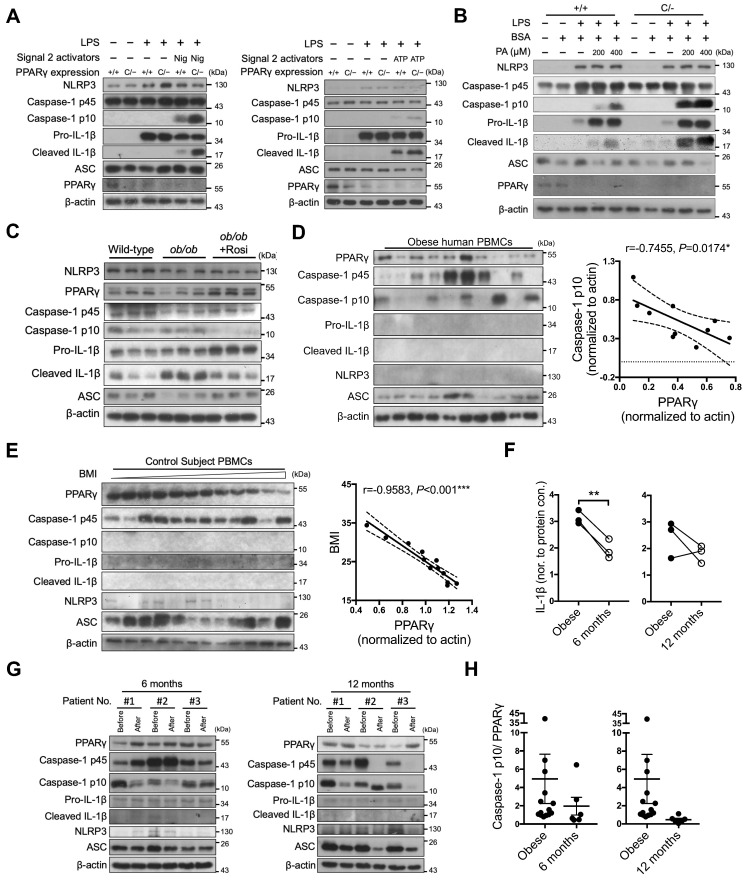
Figure 7.
PPARγ is required to limit NLRP3 inflammasome activation in mice and humans. (A-B) Immunoblot analysis of caspase-1 activation and IL-1β maturation in LPS-primed wild-type (Pparg+/+) and PpargC/- mouse peritoneal macrophages treated with (A) nigericin (Nig) and ATP, or with (B) palmitic acid (PA). (C) Immunoblot analysis of caspase-1 activation and mature IL-1β in the supernatant and NLRP3 inflammasome components and PPARγ in the cell lysates of lean control and ob/ob obese mouse peritoneal macrophages treated ex vivo with LPS and nigericin, as well as rosiglitazone, by Signal-2 exposure protocol. Each lane represents one independent experiment, and three independent experiments were included in this blot. (D-E) Immunoblot analysis of PPARγ, caspase-1 activation, mature IL-1β, NLRP3, and ASC in the cell lysates of peripheral blood mononuclear cells (PBMCs) from (D) obese and (E) control subjects. The correlation between caspase-1 activation and PPARγ level in the cell lysates of PBMCs from obese subjects. Spearman's rank correlation coefficients r and P value are provided. (F) IL-1β levels detected by ELISA in the cell lysates of PBMCs from obese subjects before and 6 (left panel) or 12 (right panel) months after weight-loss surgery. (G) Immunoblot analysis of PPARγ, caspase-1 activation, mature IL-1β, NLRP3, and ASC in the cell lysates of PBMCs from obese subjects before and 6 (left panel) or 12 (right panel) months after weight-loss surgery. (H) The ratio of caspase-1 activation to PPARγ in the cell lysates of PBMCs from obese subjects before and 6 (left panel) or 12 (right panel) months after weight-loss surgery. *P < 0.05 by paired t-test in (F). Representative blots in (A) and (B) are from three independent experiments. Experiment replicates are shown in Figure S16.