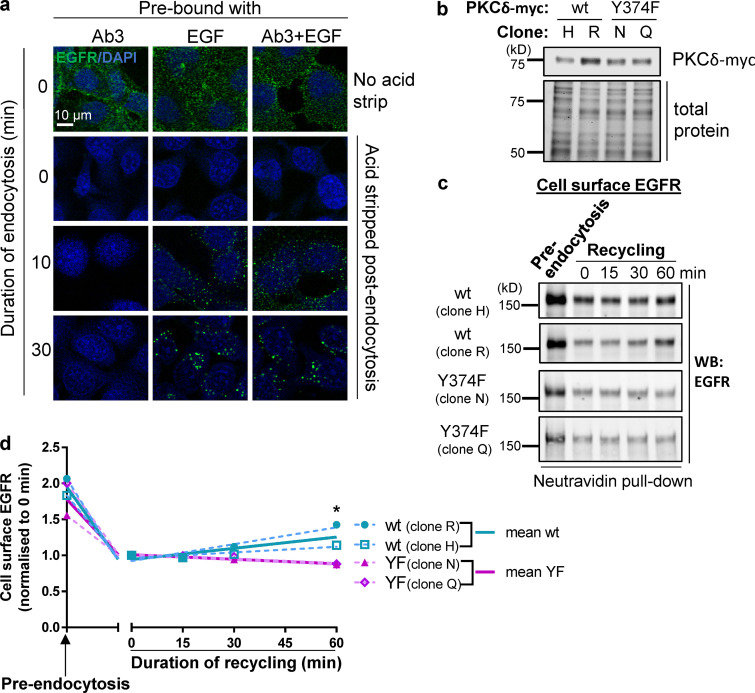
Figure S2.
Phosphorylation of Y374-PKCδ is required for EGFR recycling. (a) The EGFR-ECD antibody (Ab3) alone does not induce endocytosis. Representative immunofluorescence micrographs showing cells prebound (at 4°C) with the EGFR-ECD antibody alone and EGF (20 ng/ml) alone or with the EGFR-ECD antibody in the presence of EGF. Cells were either fixed without inducing endocytosis (0 min, no acid strip) or induced to undergo endocytosis for various times, as indicated, by incubation at 37°C, followed by an acid wash to remove EGFR-ECD antibody and EGF remaining on the cell surface (acid strip) after endocytosis. Cells were then permeabilized and incubated with secondary Ab (cells prebound with Ab3, Ab3 + EGF) or with the Ab3 antibody followed by secondary antibody (cells prebound with EGF only) to detect endocytosed EGFR. (b) Representative Western blot showing two BT-549 clones overexpressing wt-PKCδ-myc and two clones overexpressing Y374F-PKCδ-myc. (c and d) EGFR recycling requires phosphorylation of Y374-PKCδ. BT-549 cells overexpressing wt (two clones, H and R) or Y374F-PKCδ (two clones, N and Q) were either surface biotinylated before inducing endocytosis (preendocytosis) or induced to undergo a pulse of endocytosis with EGF (20 ng/ml) and cell surface biotinylated after allowing recycling to commence for the duration indicated. The total cell surface biotinylated proteins were captured using NeutrAvidin beads and amount of biotinylated EGFR detected by Western blotting (WB) with an EGFR antibody (c) and quantified (d; graphs of individual clones [dashed lines] and the means of n = 2 wt and n = 2 Y374F-PKCδ clones [solid lines] plotted using segmental nonlinear regression analysis; *, P < 0.05, multiple t tests; Prism).