Extended Data Figure 3 |. The COPI–Arf79F complex regulates stem cell survival through lipolysis and knockdown of these genes blocks lipolysis, but promotes lipid storage.
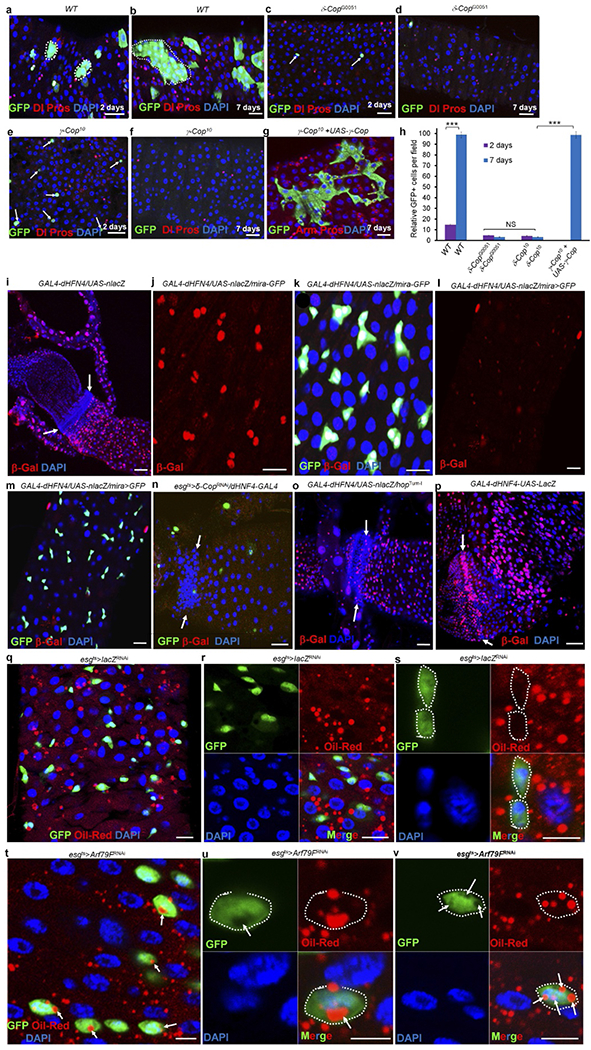
a–h, The COPI complex autonomously regulates stem cell survival. The three- or four-day-old adult female flies were heat-shocked twice with an interval of 8–12 h, at 37 °C, for 60 min to induce MARCM clones17. In wild-type clones, small GFP+ cell clusters were detected 2 d ACI (a, h; n = 33), which grew into large clusters that contained both ISCs and their differentiated progenies by 7 d ACI (b, h; n = 37). In the δ-COP mutant clones, only a few GFP+ cells were identified 2 d ACI (c, h; n = 34), and none were seen at 7 d ACI (f, h; n = 31). Similarly, only a few GFP+ cells were identified at 2 d ACI in γ-COP (e, h; n = 27) mutant clones, and none were seen at 7 d ACI (f; h; n = 34). Expressing UAS–γ-COP–GFP in γ-COP10-mutant MARCM clones (g and h; n = 31) completely rescued the stem cell death phenotype. These results suggest that the COPI complex cell-autonomously regulates stem cell survival. Dotted lines in a and b outline GFP+ clones. White arrows in c and e point to individual GFP+ cells. h, Quantification of GFP+ cells in the indicated panels. Data show the mean ± s.e.m. Statistical significance was determined by Student’s t-test, ***P < 0.0001. The posterior midguts of flies with the indicated genotypes were dissected, stained with the indicated antibodies and analysed by confocal microscopy. i–p, The lipolysis pathway is active in stem cells. To further investigate the function of lipolysis in stem cells, we investigated the expression of a lipolysis reporter (GAL4–dHFN4; UAS–nlacZ)21. In our system, this reporter showed strong β-galactosidase expression in mira-GFP-positive ISCs and RNSCs (i–k, n = 15), but not in enterocytes, enteroendocrine cells, and the quiescent HISCs of mature adult flies (i, white arrows, 3–5 days old) or in the quiescent ISCs of freshly emerged young adult flies (less than 3 days old; l and m, n = 17)49 at 29 °C culture conditions. Expressing δ-COPRNAi (esgts > δ-COPRNAi + GAL4–dHFN4; UAS–nlacZ) almost completely eliminated the reporter expression (n, n = 24), suggesting that the reporter is specifically regulated by the COPI complex. We also expressed a constitutively active form of JAK (hopTum-l) with GAL4–dHFN4; UAS–nlacZ and found that the reporter was expressed in hopTum-l-activated HISCs (o, white arrows, n = 20). The GAL4 in the reporter system is under the control of an hsp70 promoter; we heat-shocked the flies for 30 min at 37 °C 12 h before dissection and found that the reporter was strongly expressed in ISCs, RNSCs and HISCs (particularly strong in HISCs), but not in enteroendocrine cells and enterocytes (p, white arrows, n = 17). Arrows in i, n, o and p point to HISCs at the hindgut–midgut junction. q–v, Arf79F knockdown promotes lipid storage in stem cells. The genotypes of the flies in each panel were: q–s, esgts > lacZRNAi, 29 °C, 4 d (n = 30). t–v, esgts > Arf79FRNAi, 29 °C, 4 d (n = 37). The posterior midguts of flies with the indicated genotypes were dissected, stained with Oil Red O (red), anti-GFP (green) and DAPI (blue), and analysed by confocal microscopy. Dotted lines outline stem cells and white arrows point to lipid droplets in stem cells. Scale bars in a–g and i–v, 10 μm.
