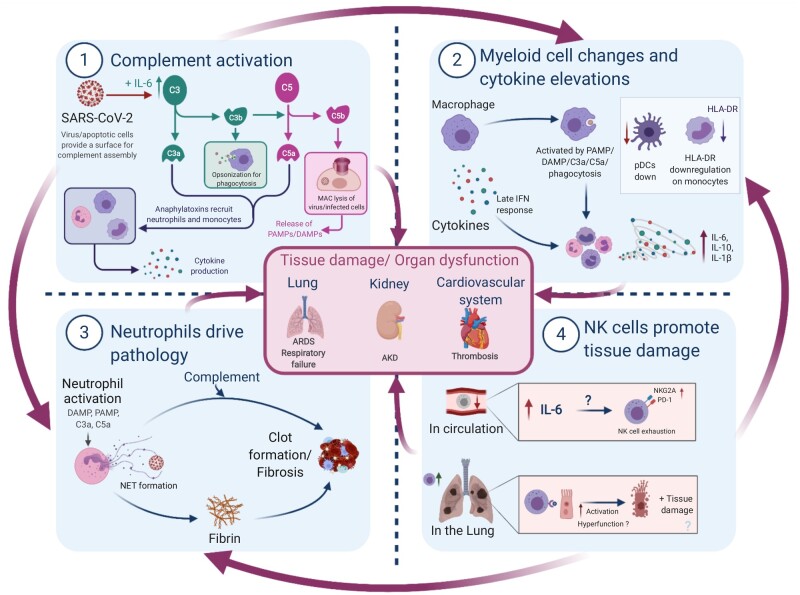
Figure 1:
Summary of innate immune-driven pathology. Graphical summary depicting the role of innate immune components in COVID-19 pathology. (1) Complement is elevated by IL-6 and likely activated by viral/apoptotic cell membranes to initiate inflammation via anaphylatoxins. (2) Macrophages can sense tissue damage (DAMPs), complement (anaphylatoxins or C3b opsonization) or virus (PAMPs) to release cytokines; direct infection delays IFN responses. Monocytes display signs of activation or anergy through HLA-DR downregulation, while anti-viral pDCs are decreased. (3) Neutrophils respond to damage, complement and cytokines by releasing NETs consisting of fibres and DNA. NETs may act as a nucleation point for complement and coagulation factors to drive thrombosis—thought to be a major driver of mortality in COVID-19. (4) NK cells are reduced in circulation and display markers of exhaustion, which is possibly driven by elevated IL-6. These cells traffic to infected lungs where they clear virus-infected cells in mild COVID-19. However, in severe disease, these cells are possibly less effective or may contribute to tissue damage and further inflammation via DAMP release from apoptotic cells, especially if phagocytes are dysfunctional. Importantly, NK cell mechanisms are still poorly understood in COVID-19. All these innate immune factors together promote the hyperinflammation, tissue damage and organ dysfunction observed in severe COVID-19, though such inflammation may be followed by immunosuppression. AKD, acute kidney disease.