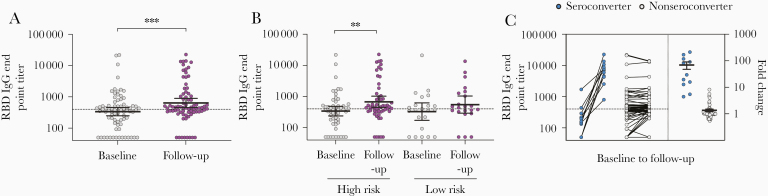
Figure 2.
SARS-CoV-2 RBD-specific IgG antibodies in HCW before and after COVID-19 patient admissions. A, The RBD-specific IgG end point titers were measured for HCW with positive or intermediate RBD screening results (n = 76) by ELISA. B, RBD-specific IgG end point titers in high-risk and low-risk HCW groups. C, HCW were divided into seroconverters (blue circle) who were seropositive and had ≥4-fold increase in IgG titers at follow-up and nonseroconverters (gray circle) who were either seronegative or had <4-fold increase in IgG titers at follow-up. The fold changes are plotted on the right y-axis with horizontal lines representing the mean with standard error of the mean. Dotted lines represent cutoffs for positive results, calculated as 3 standard deviations above the mean of the prepandemic negative sera (RBD IgG end point titer ≥400). Individuals with undetectable antibodies were assigned an end point titer of 50 for plotting and calculation purposes. End point titers were log-transformed and compared between time points in mixed-effects models with adjustment for subject variation, age, sex, and other relevant demographic factors. **P < .01, ***P < .001. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; HCW, health care workers; IgG, immunoglobulin G; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.