Abstract
Controlled human infection (CHI) models for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been proposed as a tool to accelerate the development of vaccines and drugs. Such models carry inherent risks. Participants may develop severe disease or complications after deliberate infection. Prolonged isolation may negatively impact their well-being. Through secondary infection of study personnel or participant household contacts, the experimental virus strain may cause a community outbreak. We identified risks associated with such a SARS-CoV-2 CHI model and assessed their likelihood and impact and propose strategies that mitigate these risks. In this report, we show that risks can be minimized with proper risk mitigation strategies; the residual risk, however, should be weighed carefully against the scientific and social values of such a CHI model.
Keywords: COVID-19, SARS-CoV-2, controlled human infection model, human challenge model, risk assessment
Controlled human infection models of severe acute respiratory syndrome coronavirus 2 have inherent risks to the individual study participant and to third-party contacts. Risks may be minimized with proper risk mitigation strategies.
Since the first reported cases in December 2019, coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly developed into a global pandemic. In the absence of preventive vaccines and effective treatments, the current control strategy is aimed at preventing viral transmission through behavioral changes at the population level and widespread implementation of testing, tracing, and isolation measures. Drafting of these public health guidelines is complicated by the incomplete knowledge about the transmission of the virus, the disease, and the acquisition of immunity.
An effective vaccine against SARS-CoV-2 is the key to control the COVID-19 pandemic. Currently there are >170 SARS-CoV-2 vaccine candidates in development [1]. Since it is not feasible to test all these vaccine candidates for safety and efficacy in placebo-controlled phase 3 studies or noninferiority trials with tens of thousands of patients, a rapid assessment of vaccine candidates is needed to allow multiple vaccines to supply the world market. At the same time, implementation of public health measures and potential first-generation vaccines may cause a decline in the incidence of cases, complicating phase 3 efficacy testing. Similarly, antiviral drugs, preferably active against the entire coronavirus family, will be crucial to manage new outbreaks in the long term, and rapid testing is essential.
Several investigators have proposed to develop a human experimental (challenge) model, or controlled human infection (CHI) model, for SARS-CoV-2 with a recent announcement of the initiation of such studies by Imperial College London [2]. In such models, healthy young volunteers would be experimentally infected with SARS-CoV-2 to test the efficacy of novel vaccines or drugs. For other infectious diseases such as influenza, respiratory syncytial virus, and malaria, use of these CHI models in product development has increased exponentially in recent years [3]. These models carry inherent risks and thus require thorough ethical examination. Discussions about the social values and conditions under which a SARS-CoV-2 CHI model could be ethically acceptable [4, 5] or unacceptable [6] have been published. The feasibility, potential value, and limitations of such a model have been extensively assessed by a World Health Organization (WHO) advisory group [7]. Here we have performed a comprehensive quantitative analysis of the risks related to such experiments and potential mitigation strategies to ultimately weigh risks against the potential public health value and contribute to the roadmap for SARS-CoV-2 CHI studies.
RISKS OF ACQUIRING SEVERE DISEASE
How Likely is One to Develop Severe Disease After Infection?
Experimental infection of human volunteers with SARS-CoV-2 will inherently come with a risk of causing severe COVID-19 disease. Severe disease may be defined as the need for hospitalization, including critical care and death. By combining publicly available hospitalization, intensive care unit (ICU) admission, and mortality numbers with data from nationwide seroprevalence studies, we set out to crudely estimate corresponding hospitalization, ICU admission, and death rates for different age groups in different countries (Table 1). Methods for data acquisition and calculations are provided in the Supplementary Appendix. The observed overall risk for hospitalization varies between 12.1 and 38.2 per 1000 infections, whereas the risk of ICU admission varies between 24.0 and 42.2 per 10 000 infections with death rates of 289.9–1111.2 per 100 000 infections. Rates are substantially lower for younger age groups with estimations of 0.8–3.9 per 1000, 0.9–4.5 per 10 000, and 1.2–6.1 per 100 000 infections for hospitalization, ICU admission, and death, respectively, in persons <30 years of age. Similarly, a recent publication of a comprehensive analysis of age-specific infection fatality rates found an infection fatality rate of 10 per 100 000 for persons aged 0–34 years [8]. In a detailed assessment of our own regional epidemiological data in the Netherlands, we were able to estimate a risk for hospitalization of 1 per 1000 infections for persons aged 18–29 years. None were admitted to the ICU or died at the time of the query. These rates are lower than initial estimates [9] upon which subsequent ethical assessments for use of CHI models were based [4, 5]. Moreover, these data included persons with comorbidities, overestimating the risk in healthy individuals. In addition, our estimates included young children, which in most countries form a large proportion of all persons <30 years of age hospitalized, further overestimating the risk. Furthermore, for most countries the observed seroprevalence rates are probably an underestimation of the true infection rate for several reasons: (1) In most reports, only immunoglobulin G was measured; therefore, immunoglobulin M positives or immunoglobulin A positives were missed; (2) seroconversion may not occur in everyone; and (3) antibodies may wane [10, 11]. To put our estimations in perspective, we found risks between 1.2 and 6.1 deaths per 100 000 infections in people aged <30 years, which is comparable to the risk of road deaths, which ranged from 3.0 to 5.1 per 100 000 inhabitants [12] in Belgium, Denmark, the Netherlands, Spain, and Sweden in 2018. In fact, in the Netherlands, the number of road deaths in this age groups was 23 per 100 000 for all road participants, 3.8 per 100 000 for bicycle riders, and 1.0 per 100 000 for pedestrians [13]. Live kidney donation reportedly had a 3-month and 12-month mortality risk of 3.0 and 6.0 per 10 000 donors aged 18–39 years, respectively [14]. Of course, our crudely calculated rates should be interpreted with caution as epidemiological data may not fully reflect risks in challenge studies due to differences in, for example, the initial infecting viral dose.
Table 1.
Age-Stratified Ranges of Hospitalization, Intensive Care Unit Admission, and Death Rates After Infection
| Age Group | Hospitalizations per 1000 Infections | ICU Admissions per 10 000 Infections | Deaths per 100 000 Infections |
|---|---|---|---|
| <30 y | 0.8–3.9 | 0.9–4.5 | 1.2–6.1 |
| <40 y | 1.3–7.4 | 2.0–7.1 | 3.1–12.0 |
| <50 y | 2.5–11.7 | 4.7–13.3 | 7.2–24.9 |
| <60 y | 4.8–17.2 | 8.8–26.3 | 9.6–57.9 |
| <70 y | 7.3–22.9 | 14.8–37.7 | 65.3–137.3 |
| All | 12.1–38.2 | 24.0–42.2 | 289.9–1111.2 |
For each age group, ranges from point estimates of hospitalization, ICU admissions, and death rates are shown. Crude point estimates were calculated from reported events and seroprevalence rates for Belgium, Denmark, the Netherlands, Spain, and Sweden (see Supplementary Data for calculations).
Abbreviation: ICU, intensive care unit.
Factors Associated With Disease Severity
The degree of disease severity is closely related with age, male sex, obesity, and possibly smoking [15], and the presence of chronic major comorbidities [16–19]. Between 70% and 80% of hospitalized patients reportedly had 1 or more comorbidities [16], of which chronic cardiac disease, uncomplicated diabetes mellitus, nonasthmatic chronic pulmonary disease, and chronic kidney disease are most common. Rare putative loss-of-function variants of the X-chromosomal TLR7 gene were also associated with severe disease in young males [20]. The risk of severe disease may also be determined by the initial infecting viral dose. This could explain why dentists, otorhinolaryngologists, and anesthesiologists are at higher risk of severe COVID-19 [21, 22]. Based on current findings from an animal challenge study [23], an influenza CHI study [24], and a human epidemiological study [25], we may similarly assume a dose-dependent clinical response to infection with SARS-CoV-2. There are concerns that suboptimal antibody acquisition after a primary SARS-CoV-2 infection may enhance disease severity of a subsequent infection with SARS-CoV-2 by so-called antibody-dependent enhancement (ADE), as has been suggested to occur after vaccination [26]. Whether ADE occurs after SARS-CoV-2 infection remains to be elucidated; 1 possible case has been described recently [27]. However, the potential of this phenomenon should be recognized and potential mitigation strategies explored.
Risk Mitigation Strategies
The most evident mitigation strategy would be the availability of a rescue treatment option that would cure 100% of individuals, preferably after study endpoints are met. Two pharmaceutical treatments, remdesivir and dexamethasone, were shown to be effective in severely affected patients [28, 29], although this has been contested in more recent data for remdesivir [30]. More clinical trials evaluating the efficacy of immunomodulatory drugs are ongoing [31]. Antibody therapy may be another treatment modality in the future [32, 33]. Incorrect timing of treatment initiation may explain why clinical trials of convalescent plasma therapy were seemingly ineffective [34, 35]. Early clinical trials of antibody therapy with specific neutralizing antibodies are underway [36, 37]. Proper dosing of anticoagulation in hospitalized patients with COVID-19 is common practice, despite the lack of proven efficacy through randomized trials, and may reduce disease severity risk caused by a hypercoagulable state [38].
Selection of low-risk participants could further reduce the risk for severe disease. Healthy young adult volunteers, preferably nonsmokers aged 18–29 years, who do not have any of the above-mentioned risk factors, including comorbidities and obesity, should be selected. The resulting probability of severe or critical disease and death after infection would then be a fraction of what we estimated it to be for the general population (Table 1), for example, 60% lower rates if we assume that 40% of young hospitalized adults had comorbidities and/or obesity. Of course, selection of volunteers will also compromise extrapolation of results to the field.
Because of the uncertainty around the possible existence of ADE, exclusion of participants with previous exposure seems preferred. However, if ADE exists for SARS-CoV-2, participants could potentially have an increased risk of severe disease after the trial. The longevity of such antibody-mediated effects is currently unknown but may be short [10, 11].
To further mitigate risk, the SARS-CoV-2 challenge virus may be attenuated. Currently, very little is known about mutations and their relation to virulence. However, as our knowledge on the SARS-CoV-2 virus increases, genetic engineering will allow for the design of potentially attenuated viral strains.
Besides attenuation, the challenge virus inoculum dose can be carefully titrated from the lowest possible dose up until a minimal viral inoculum dose that leads to relevant study endpoints. In addition, the study endpoint that can be achieved with the lowest risk (eg, measurable viral shedding or observable clinical symptoms) can be chosen.
RISKS OF (LONG-TERM) COMPLICATIONS
What Are Potential Complications After Getting Infected?
Severe COVID-19 is characterized by pneumonia or acute respiratory distress syndrome (ARDS) associated with dysregulated and excessive cytokine release with subsequent multiorgan failure [39]. Survival of critical illness may be accompanied by long-term and sometimes permanent impairments in cognition, psychological health, and physical functioning [40]. Infection of the lungs has led to concerns of long-term pulmonary function decline after COVID-19, similar to what was observed after the severe acute respiratory syndrome outbreak [41], although this is mostly seen after ARDS and thus in patients who had severe symptoms [42]. Debilitating symptoms such as fatigue and headache for weeks or months have been reported after less severe illness—a condition now referred to as “postacute COVID-19” or “long-COVID” [43]. Symptoms lasting for more than 4, 8, and 12 weeks were observed in 13.3%, 4.5%, and 2.3%, respectively, of persons who filled in the COVID Symptom Study app [44], although numbers in those aged <30 years were much lower, at 0.9% for males and 1.4% for females at 12 weeks (personal communication, Sudre and Steves) [44]. In this study, which inherently excludes asymptomatic persons, occurrence of long-COVID was associated with female sex, higher age, and higher body mass index, and 31.5% reportedly required hospital assessment during the acute period. The frequency of long-COVID in young and healthy (nonhospitalized) low-risk patients is currently unclear, but likely to be lower. Venous thromboembolism is frequently observed in seriously ill patients [45, 46]. Ischemic stroke was observed in 0.9% and 4.6% of hospitalized COVID-19 patients in New York [47] and Wuhan [48], respectively; however, the true effect of the association between COVID-19 and ischemic stroke remains uncertain [49]. A causal link with SARS-CoV-2 infection is less clear for certain hyperinflammatory or autoimmune complications temporally associated with COVID-19, of which the most well-known is the pediatric inflammatory multisystem syndrome [50]. Other potential complications observed are rhabdomyolysis, autoimmune hemolytic anemia, immune thrombocytopenia, Guillain-Barré syndrome, and subacute thyroiditis [51]. It should be noted that most of the above-mentioned complications were observed in hospitalized patients. The probability of acquiring such complications is therefore a minor fraction of the probability of hospitalization after SARS-CoV-2 infection. There are concerns that occult cardiac inflammation occurs in a substantial portion of nonhospitalized patients [52]; however, the exact clinical consequences of these findings are still unclear. Last, while seemingly rare complications (eg, Guillain-Barré syndrome) have been observed, the true incidence of such complications is unknown. It might occur more often than perceived, but a direct connection between the complication and an earlier undetected SARS-CoV-2 infection may not be apparent. Future studies, particularly in the primary care setting, are needed to properly evaluate incidence rates.
Risk Mitigation Strategies
The risk of developing complications or long-lasting symptoms is probably mostly mitigated by minimizing the risk of developing severe disease. In addition, awareness by study physicians of potential (rare) complications, together with close observation of participants’ well-being during infection and after infection, may lead to early recognition and treatment should these complications occur. Regardless of mitigation strategies, proper compensation methods for volunteers in case of long-term sequelae or severe disease should be in place, either through mandatory clinical trial insurance or additional compensation methods.
THIRD-PARTY RISKS
Should We Be Afraid of Community Transmission?
Persons most at risk of getting secondarily infected by study participants are study personnel and participants’ household contacts. In theory, infected study personnel or household contacts could introduce the experimental strain into the community. Community transmission of an experimental strain may fuel public distrust in science in general and CHI studies in particular. The likelihood, however, of infecting study personnel seems low with proper implementation of infection prevention and control measures, including use of personal protective equipment (PPE). Nosocomial transmission of virus between healthcare workers and from inpatients to healthcare workers was almost not observed in hospitals that implemented proper infection control measures, including use of adequate PPE [53, 54]. These findings are supported by a study performing whole-genome sequencing of SARS-CoV-2 in clinical samples taken from healthcare workers and patients in 3 hospitals in the Netherlands, providing evidence for community exposure rather than nosocomial transmission [55]. In contrast, secondary transmission to household contacts is likely to occur if infected persons are not properly isolated [56, 57]. This becomes especially problematic when participants decide to prematurely opt out from an ongoing challenge study while they are still shedding virus.
Risk Mitigation Strategies
Proper implementation of infection control measures and use of PPE will be of utmost importance to reduce the risk of transmission to research staff. Repeated screening (eg, every other day) of research staff who have direct contact with infected participants for at least 2 weeks after their latest high-risk contact, irrespective of symptoms, may lead to timely detection of secondary infection should it occur. The impact of transmission to research staff may be mitigated if only staff with low risk for the development of severe disease are allowed in proximity of participants. Participants will have to stay inpatient until nasal shedding of viable infectious virus has stopped. Because polymerase chain reaction (PCR) positivity does not always reflect the presence of viable viral particles and shedding may persist over a longer time, the use of viral culture seems the most appropriate parameter. Alternatively, short-term viral culture with immunofluorescence virus detection methods can be considered. Negative results from nasal swab or throat swabs on several consecutive days would be needed to allow for safe lifting of isolation measures. To reduce the impact of community transmission, local public health authorities should be notified to anticipate contact tracing and isolation measures, should they be needed. Last, to mitigate the risk that participants infect others because they decided to leave the quarantine facility prematurely, participants will have to agree to be subjected to legally enforceable isolation.
RISKS ASSOCIATED WITH ISOLATION
What Is the Negative Effect of Participant Quarantine?
Sudden quarantine or isolation is associated with negative psychological impact driven by different stressors such as duration of quarantine/isolation, frustration and boredom, inadequate information, financial loss, and stigmatization from others [58]. It is probably unlikely that these stressors play a role in participants who are expecting a certain study-related duration of isolation; however, unforeseen prolongation of isolation (eg, because of prolonged detection of viral RNA shedding) could induce negative psychological impact in participants including unexpected financial losses.
Risk Mitigation Strategies
While effects of isolation on the mental health of participants is unlikely, performing a mental health screening could mitigate the risk of mental health effects of unexpected prolonged isolation should this occur. Any losses of income due to unexpected prolonged isolation will have to be compensated as to reduce the impact of this risk. The duration of isolation may be decreased when discharge criteria are based on the detection of viable (cultured) virus instead of PCR negativity. Viable virus was unlikely to be cultured after 9 days in patients with mild disease, whereas patients with severe or critical disease had culturable virus shedding for a median duration of 8 days (interquartile range, 5–11 days [range, 0–20 days]) [59]. As such, for SARS-CoV-2 CHI study participants, an isolation duration of approximately 2 weeks may be expected when discharge criteria are based on the detection of viable virus. Using PCR negativity as a discharge criterion could substantially prolong isolation for days to weeks as compared to basing discharge on viral culture assays [60, 61].
OVERALL RISK ASSESSMENT
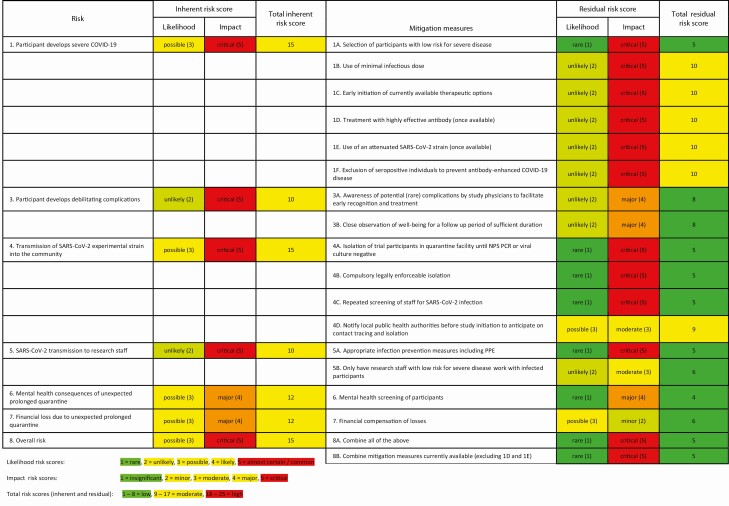
In Table 2, we provide an overview of identified risks and risk mitigation measures. The level of risk (inherent risk score) and effectiveness of proposed risk mitigation measures (residual risk score) was determined by consensus between the authors. Likelihood was scored as almost certain/common, 5; likely, 4; possible, 3; unlikely, 2; rare, 1. Impact was scored as critical, 5; major, 4; moderate, 3; minor, 2; insignificant, 1. Resulting residual risk scores (product of likelihood and impact) were separated into 3 categories: risks of 18–25 were considered high, resulting risk scores in the range of 9–17 were considered moderate, and resulting scores of 1–8 were considered low. We show that proposed mitigation measures may influence the residual risk score to different degrees, but overall, and especially when combined, risks may be mitigated to acceptable levels.
Table 2.
Risk Assessment Table
For each risk, likelihood and impact scores (inherent risk score) were determined; the product of both determines the total inherent risk score. For each risk, at least 1 mitigation measure was proposed. Each mitigation measure may influence the residual risk score to a different degree. For example, the total residual risk scores for mitigation 1A and 1F are 5 and 10, respectively, meaning that measure 1A is thought to be more effective at reducing risk 1 (development of severe disease) than 1F.
Abbreviations: COVID-19, coronavirus disease 2019; NPS, nasopharyngeal swab; PCR, polymerase chain reaction; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
We should note that the proposed mitigation strategies would ultimately result in the selection of study participants who do not necessarily reflect the target population for SARS-CoV-2 vaccines or therapeutics. The resulting loss of scientific value should be taken into consideration, as has been done by the WHO advisory group, which concluded that such a model might still be useful in order to select among multiple vaccines, investigate correlates of protection, investigate viral dose–clinical severity relationships, and helping to estimate transmission risks [7].
In summary, in this report, we show that risks associated with the experimental infection of human volunteers with SARS-CoV-2 can be minimized if proper mitigation strategies are put in place, leaving a residual risk that should be weighed carefully against the scientific and social values of such a human SARS-CoV-2 model.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Prof. Dr. Hans Zaaijer (Sanquin Research, the Netherlands) for providing us with the age-specific (18–29 years) seroprevalence number of “security region” Hollands-Midden and Haaglanden.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Kommenda N, Hulley-Jones F. Covid vaccine tracker: when will we have a coronavirus vaccine?2020. Available at: https://www.theguardian.com/world/ng-interactive/2020/nov/10/covid-vaccine-tracker-when-will-a-coronavirus-vaccine-be-ready. Accessed 17 August 2020.
- 2.Kirby T. COVID-19 human challenge studies in the UK [manuscript published online ahead of print 30 October 2020]. Lancet Resp Med 2020. doi:10.1016/S2213-2600(20)30518-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roestenberg M, Hoogerwerf MA, Ferreira DM, Mordmüller B, Yazdanbakhsh M. Experimental infection of human volunteers. Lancet Infect Dis 2018; 18:e312–22. [DOI] [PubMed] [Google Scholar]
- 4.Shah SK, Miller FG, Darton TC, et al. . Ethics of controlled human infection to address COVID-19. Science 2020; 368:832–4. [DOI] [PubMed] [Google Scholar]
- 5.Jamrozik E, Selgelid MJ. COVID-19 human challenge studies: ethical issues. Lancet Infect Dis 2020; 20:e198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn JP, Henry LM, Mastroianni AC, Chen WH, Macklin R. Opinion: for now, it’s unethical to use human challenge studies for SARS-CoV-2 vaccine development. Proc Natl Acad Sci U S A 2020; 117:28538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Advisory Group on Human Challenge Studies. Feasibility, potential value and limitations of establishing a closely monitored challenge model of experimental COVID-19 infection and illness in healthy young adult volunteers.2020. Available at: https://www.who.int/publications/m/item/feasibility-potential-value-and-limitations-of-establishing-a-closely-monitored-challenge-model-of-experimental-covid-19-infection-and-illness-in-healthy-young-adult-volunteers. Accessed 16 July 2020.
- 8.Levin AT, Cochran KB, Walsh SP. Assessing the age specificity of infection fatality rates for COVID-19: meta-analysis and public policy implications.medRxiv [Preprint]. Posted online 31 October; 2020. doi:10.1101/2020.07.23.20160895. [Google Scholar]
- 9.Verity R, Okell LC, Dorigatti I, et al. . Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020; 20:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seow J, Graham C, Merrick B, et al. . Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv [Preprint]. Posted online 11 July 2020. doi:2020.07.09.20148429. [Google Scholar]
- 11.Long QX, Tang XJ, Shi QL, et al. . Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
- 12.Stipdonk H, Sanz-Villegas MT, Thomas P, et al. . Ranking EU progress on road safety: 13th road safety performance index report.2019. Available at: https://etsc.eu/wp-content/uploads/AR_2019-Final.pdf. Accessed 16 July 2020.
- 13.StatLine–Overledenen. Doden door verkeersongeval in Nederland, wijze van deelname. Available at: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/71936ned/table?ts=1592135018433. Accessed 16 July 2020.
- 14.Segev DL, Muzaale AD, Caffo BS, et al. . Perioperative mortality and long-term survival following live kidney donation. JAMA 2010; 303:959–66. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Smoking and COVID-19. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Smoking-2020.2. Accessed 21 August 2020.
- 16.Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C Investigators . Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson EJ, Walker AJ, Bhaskaran K, et al. . Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S, Hirsch JS, Narasimhan M, et al. ; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Made CI, Simons A, Schuurs-Hoeijmakers J, et al. . Presence of genetic variants among young men with severe COVID-19. JAMA 2020; 324:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ing EB, Xu QA, Salimi A, Torun N. Physician deaths from corona virus (COVID-19) disease. Occup Med (Lond) 2020; 70:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work. 2020; 11:262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan KA, Bewley KR, Fotheringham SA, et al. . Dose-dependent response to infection with SARS-CoV-2 in the ferret model: evidence of protection to re-challenge. bioRxiv [Preprint]. Posted online 29 May 2020. doi:2020.05.29.123810. [Google Scholar]
- 24.Han A, Czajkowski LM, Donaldson A, et al. . A dose-finding study of a wild-type influenza A(H3N2) virus in a healthy volunteer human challenge model. Clin Infect Dis 2019; 69:2082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guallar MP, Meiriño R, Donat-Vargas C, Corral O, Jouvé N, Soriano V. Inoculum at the time of SARS-CoV-2 exposure and risk of disease severity. Int J Infect Dis 2020; 97:290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol 2020; 20:339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillett RL, Sevinsky JR, Hartley PD, et al. . Genomic evidence for reinfection with SARS-CoV-2: a case study [manuscript published online ahead of print 12 October 2020]. Lancet Infect Dis 2020. doi:10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beigel JH, Tomashek KM, Dodd LE, et al. . Remdesivir for the treatment of Covid-19—preliminary report. New Engl J Med 2020; 383:1813–26. [DOI] [PubMed] [Google Scholar]
- 29.Horby P, Lim WS, Emberson J, et al. . Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv [Preprint]. Posted online 22 June 2020. doi:2020.06.22.20137273. [Google Scholar]
- 30.Pan H, Peto R, Abdool Karim Q, et al. . Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. medRxiv [Preprint]. Posted online 15 October 2020. doi: 10.1101/2020.10.15.20209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alzghari SK, Acuña VS. Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol 2020; 127:104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham J. Passive antibody therapy in COVID-19. Nat Rev Immunol 2020; 20:401–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marovich M, Mascola JR, Cohen MS. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA 2020; 324:131–2. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Li L, Zhang W, et al. . Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020; 324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gharbharan A, Jordans CCE, Geurtsvankessel C, et al.Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv [Preprint]. Posted online 3 July 2020. doi: 10.1101/2020.07.01.20139857. [DOI] [Google Scholar]
- 36.ClinicalTrials.gov. Safety, tolerability, and efficacy of anti-spike (S) SARS-CoV-2 monoclonal antibodies for hospitalized adult patients with COVID-19.2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04426695. Accessed 16 July 2020.
- 37.ClinicalTrials.gov. A study of LY3819253 (LY-CoV555) in participants with early mild to moderate COVID-19 illness. Available at: https://clinicaltrials.gov/ct2/show/NCT04427501. Accessed 16 July 2020.
- 38.UpToDate. Coronavirus disease 2019 (COVID-19): hypercoagulability. Available at: https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-hypercoagulability?topicRef=127481&source=see_link#H636846847. Accessed 17 August 2020.
- 39.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “cytokine storm”’ in COVID-19. J Infect 2020; 80:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med 2017; 5:90–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P, Li J, Liu H, et al. . Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spagnolo P, Balestro E, Aliberti S, et al. . Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med 2020; 8:750–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ 2020; 370:m3026. [DOI] [PubMed] [Google Scholar]
- 44.Sudre CH, Murray B, Varsavsky T, et al. . Attributes and predictors of long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv [Preprint]. Posted online 21 October 2020. doi:2020.10.19.20214494. [Google Scholar]
- 45.Klok FA, Kruip MJHA, van der Meer NJM, et al. . Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020; 191:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Middeldorp S, Coppens M, van Haaps TF, et al. . Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaghi S, Ishida K, Torres J, et al. . SARS-CoV-2 and stroke in a New York healthcare system. Stroke 2020; 51:2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Li M, Wang M, et al. . Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 2020; 5:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsivgoulis G, Katsanos AH, Ornello R, Sacco S. Ischemic stroke epidemiology during the COVID-19 pandemic: navigating uncharted waters with changing tides. Stroke 2020; 51:1924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beeching NJ, Fletcher TE, Fowler R. Coronavirus disease 2019 (COVID-19).2020. Available at: https://bestpractice.bmj.com/topics/en-us/3000168/complications. Accessed 16 July 2020.
- 52.Puntmann VO, Carerj ML, Wieters I, et al. . Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durante-Mangoni E, Andini R, Bertolino L, et al. . Low rate of severe acute respiratory syndrome coronavirus 2 spread among health-care personnel using ordinary personal protection equipment in a medium-incidence setting. Clin Microbiol Infect 2020; 26:1269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong SCY, Kwong RTS, Wu TC, et al. . Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 2020; 105:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sikkema RS, Pas SD, Nieuwenhuijse DF, et al. . COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis 2020; 20:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jing QL, Liu MJ, Zhang ZB, et al. . Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bi Q, Wu Y, Mei S, et al. . Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks SK, Webster RK, Smith LE, et al. . The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020; 395:912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization. Criteria for releasing COVID-19 patients from isolation.2020. Available at: https://www.who.int/publications/i/item/criteria-for-releasing-covid-19-patients-from-isolation. Accessed 16 July 2020.
- 60.Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 61.Bullard J, Dust K, Funk D, et al. . Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis 2020; 71:2663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.