Figure EV4. In vivo efficacy of EP4 antagonist (TP‐16) combined with immune checkpoint blockade.
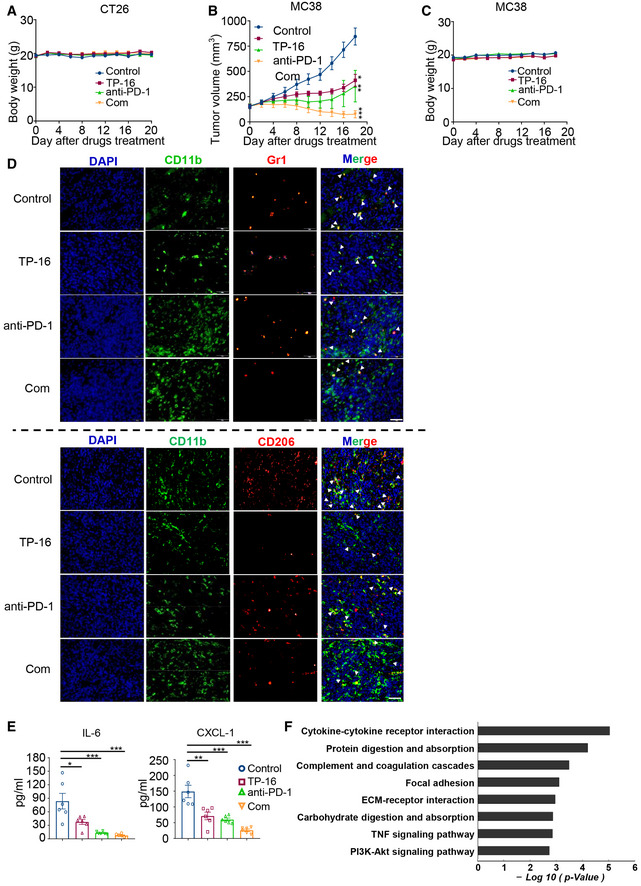
- Body weight of mice (CT26 syngeneic tumor model).
- The growth of MC38 tumors treated with vehicle, TP‐16 (75 mg/kg, po, daily), anti‐PD‐1 (50 μg, ip, twice weekly), and TP‐16 combined with anti‐PD‐1 antibody (n = 8 per group). Data are presented as mean ± SEM. One‐way ANOVA and Tukey's multiple comparison test were performed; *P < 0.05; **P < 0.01; ***P < 0.001.
- Body weight of mice (MC38 syngeneic tumor model).
- Representative immunofluorescence staining images of tumor sections from CT26 tumor‐bearing mice stained for myeloid‐derived suppressor cells (MDSCs; CD11b+Gr1+, upper panel) and M2 macrophages (CD11b+CD206+, lower panel). Scale bars, 50 μm.
- Cytokines (IL‐6 and CXCL1) in the peripheral blood of CT26 tumor‐bearing mice treated as indicated were measured by enzyme‐linked immunosorbent assay (ELISA) on the last day of treatment (n = 6). One‐way analysis of variance (ANOVA) and Turkey post hoc test were performed, *P < 0.05, **P < 0.01; ***P < 0.001.
- Pathway enrichment was analyzed based on the subsets of differentially expressed genes influenced by the combination therapy of TP‐16 and anti‐PD‐1 antibody.
Source data are available online for this figure.
