Abstract
The liver is a “front line” in the homeostatic defenses against variation in nutrient intake. It orchestrates metabolic responses to feeding by secreting factors essential for maintaining metabolic homeostasis, converting carbohydrates to triglycerides for storage, and releasing lipids packaged as lipoproteins for distribution to other tissues. Between meals, it provides fuel to the body by releasing glucose produced from glucogenic precursors and ketones from fatty acids and ketogenic amino acids. Modern diets enriched in sugars and saturated fats increase lipid accumulation in hepatocytes (nonalcoholic fatty liver disease). If untreated, this can progress to liver inflammation (nonalcoholic steatohepatitis), fibrosis, cirrhosis, and hepatocellular carcinoma. Dysregulation of liver metabolism is also relatively common in modern societies. Increased hepatic glucose production underlies fasting hyperglycemia that defines type 2 diabetes, while increased production of atherogenic, large, triglyceride-rich, very low-density lipoproteins raises the risk of cardiovascular disease. Evidence has accrued of a strong connection between meal timing, the liver clock, and metabolic homeostasis. Metabolic programming of the liver transcriptome and posttranslation modifications of proteins is strongly influenced by the daily rhythms in nutrient intake governed by the circadian clock. Importantly, whereas cell-autonomous clocks have been identified in the liver, the complete circadian programing of the liver transcriptome and posttranslational modifications of essential metabolic proteins is strongly dependent on nutrient flux and circadian signals from outside the liver. The purpose of this review is to provide a basic understanding of liver circadian physiology, drawing attention to recent research on the relationships between circadian biology and liver function.
Keywords: lipid metabolism, glucose metabolism, circadian rhythm, food intake, diabetes, dyslipidemia
Combining diets enriched with refined sugar and saturated fat with sedentary behavior often leads to obesity, metabolic dysregulation, and reduced life expectancy (1). Indeed, as middle age approaches most of us should be assessed for the modifiable risk factors for obesity-related comorbidities (abdominal obesity, impaired fasting blood glucose, atherogenic dyslipidemia, and hypertension). There is however a growing realization that excessive accumulation of fat in the liver is an independent predictor of cardiometabolic risk. Nonalcoholic fatty liver disease (NAFLD) occurs in 25% of the population (2, 3). NAFLD increases risk for cardiovascular disease and type 2 diabetes, and also for progression to more severe forms of liver disease. Inflammation of the liver (nonalcoholic steatohepatitis, NASH) is associated with an even greater risk of disease progression and morbidity and is estimated to afflict 1% to 6% of the population (2, 3). Close examination of liver function with a goal to reduce fat accumulation is thus relevant to risk reduction and healthy aging. Recent research suggests that the systems governing circadian rhythms and the metabolic functions of the liver are intrinsically linked. Moreover, some of this research suggests that implementing clinical treatments based on circadian physiology may be useful for treating metabolic disorders that increase risk of NAFLD (4-7). The goal of this review is to summarize recent literature that focuses on the interconnection between circadian rhythm and hepatic physiology/pathology, particularly in relation to NAFLD driven by diet and obesity.
The Core Clock Links Rhythms in Nutrient Flux to the Transcriptome
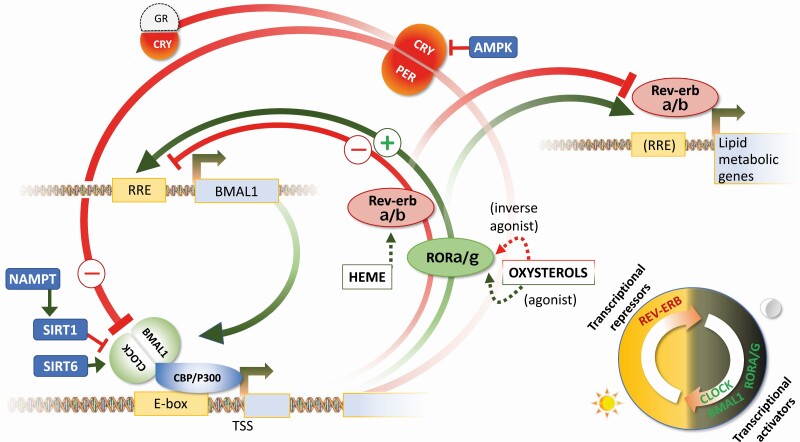
At a cellular level, circadian rhythms are maintained by a core group of transcription factors (TF) that function as either activators or repressors of gene transcription, creating an autoregulatory feedback loop that interacts with cellular sensors of the metabolic state (Fig. 1) (8). The “core” of the clock is composed of a heterodimer composed of BMAL1 (brain or muscle ARNT-like 1, also known as aryl hydrocarbon receptor nuclear translocator-like protein 1, or ARNTL) and either CLOCK (circadian locomotor output cycles kaput) or the CLOCK paralog NPAS2 (neuronal PAS domain protein 2). Mice lacking functional BMAL1 or both CLOCK paralogs (Clock–/–; Npas2–/–) do not maintain rhythms in locomotor behavior in the absence of light cues, indicating that these heterodimers are essential components of the mammalian circadian pacemaker (9, 10). BMAL1:CLOCK heterodimers interact with other tissue-specific transcription factors to activate gene transcription (11). The negative feedback loop involves 2 processes. BMAL1:CLOCK heterodimers activate the transcription of Rev-erbα and Rev-erbβ, which are transcriptional repressors that then feedback to suppress Bmal1 transcription. The phenotype of mice lacking either or both Rev-erbα and Rev-erbβ genes indicates the 2 proteins collaborate in regulating the activity of mammalian circadian pacemaker (12, 13). Rhythms in the expression of BMAL1 are driven by competition between the Rev-erb proteins and TF belonging to the retinoic acid–related orphan receptor (ROR) family (14). BMAL1:CLOCK heterodimers also activate the transcription of the Period (Per) and Cryptochrome (Cry) genes, forming heterodimers that inhibit the transcriptional activity of BMAL1:CLOCK proteins (8).
Figure 1.
Core clock feedback loop and interactors. The core clock is composed of transcriptional activators (green) and suppressors (red). The heterodimer of the basic helix-loop-helix transcription factors (BMAL1 and either CLOCK or the clock paralog NPAS2, which is not shown here) represents the forward limb of the clock in mammalian cells. BMAL1:CLOCK heterodimers bind to genomic enhancer elements, activating the transcription of circadian clock output genes during the dark period in nocturnal rodents. There are 2 repressor pathways which are active during the lights-on period. Increased transcription and activity of the transcriptional repressors Rev-erbα and Rev-erbβ results in the suppression of BMAL1, constituting a feedback loop that inhibits the forward limb. The second pathway involves heterodimers of cryptochrome (CRY) and period (PER) proteins. This heterodimer represses the transcriptional activity of the BMAL1:CLOCK/NPAS2 heterodimers. Competition for binding to ROR/REV-ERB Response Element (RRE) between the Rev-erb transcriptional repressors with RORα/γ, which are transcriptional activators, contributes to rhythms in the period of transcriptional activation or repression of large clusters of genes involved in metabolic processes. Examples of how the activity of this clock is modulated by nutrient sensors are included. Several of the transcription factors contain a Per-Arnt-Sim (PAS) domain that is responsive to metabolites, xenobiotics, and oxygen levels. SIRT1 suppresses transcriptional activity of BMAL1:CLOCK heterodimers, providing a link between the core clock to nicotinamide adenine dinucleotide (NAD+) biosynthesis by nicotinamide phosphoribosyl transferase (NAMPT). NAD acts as a coenzyme in oxygen-reduction (redox) reactions and is a substrate for Sirtuins (SIRT) that use NAD+ to remove acetyl groups from proteins. adenosine monophosphate–activated protein kinase C (AMPK) is a cellular energy sensor that regulates clock activity through phosphorylating and destabilizing CRY and PER proteins. CRY proteins also form ligand-dependent heterodimers with glucocorticoid receptors (GRs) to repress their activity. This is a simplified schematic and does not include epigenetic modifiers that interact with the core clock genes to affect rhythms in the expression of thousands of genes.
The 24-hour cycle in which periods of vigilance, foraging, and feeding behavior are interspersed between bouts of sleep that we experience is orchestrated by a “master clock” in populations of neurons in the suprachiasmatic nucleus of the hypothalamus (SCN) (8) (Fig. 2). Light signals received by the retina, and transmitted to the SCN by the retinohypothalamic tract, is the primary zeitgeber (“time-giver”) for our master clock. The core elements of the circadian clock are however expressed ubiquitously, with strong circadian profiles observed in the transcriptomes of most tissues (8, 15, 16).
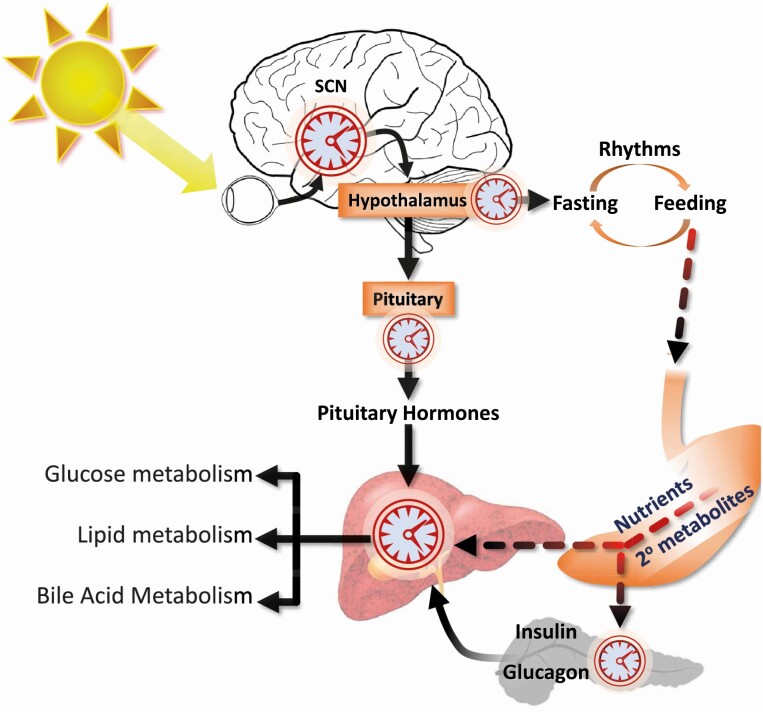
Figure 2.
Clock hierarchy in the nervous system and periphery. The master clock in the suprachiasmatic nucleus of the hypothalamus (SCN) receives light signals detected by retinal ganglion cells via the retina through the retinohypothalamic tract. Lesioning the SCN in rodents eliminates circadian rhythms in activity and core body temperature. The hypothalamus is a critical relay center that receives signals from the SCN and regulates the expression of circadian cycles of sleep and wakefulness. The hypothalamus also relays signals from the SCN to regulate autonomic and pituitary outputs that govern peripheral metabolism. Importantly, hypothalamic “fasting responsive” neurons coexpressing neuropeptide Y (Npy) and agouti-related peptide (AgRP) are responsible for the expression of rhythms in feeding behavior. These neurons possess their own innate clock that regulates synaptic activity and response to endocrine signals of fed-fasting condition. The influx of nutrients and secondary metabolites resulting from digestive processes from the digestive tract has direct and indirect effects on the liver clock. The activity of clock protein expressed in the liver is regulated by the influx of nutrients, secondary metabolites, and xenobiotics found in the diet. The indirect impact of nutrients and secondary metabolites resulting from digestive processes includes the release of pancreatic hormones, which also have expression regulated by clocks.
The “peripheral” clock in the liver resynchronizes during phase shifts in nutrient availability and appears to be independent of the master clock in the SCN (17-20). The mechanisms involved in the synchronization of the liver clock to phase shifts of nutrient intake are still unclear and are unlikely to involve a single factor or pathway. Indeed, most of the core elements of the clock possess innate nutrient-sensing mechanisms. The BMAL1, CLOCK, PER, and NPAS2 proteins all contain Per-Arnt-Sim (PAS) domain motifs that can function as sensors of a range of cellular metabolic conditions including oxygen levels and xenobiotics. The REV-ERB and ROR proteins contain ligand-binding domains that are responsive to cellular levels of heme and intermediates in cholesterol metabolism, respectively (21). Other cellular sensors of cellular metabolic state also interact with core clock proteins (see Fig. 1). This list includes members of the Sirtuin family (SIRT1, SIRT6) that respond to cellular stress, and adenosine monophosphate–activated protein kinase C (AMPK), which functions as cellular energy sensors (8). Each cell thus has the capacity to respond autonomously to the flow of nutrients throughout the body. However, the liver clock is also regulated by endocrine signals from other specialized cell types whose activity are also controlled by circadian oscillators (Fig. 2) (8). Combinations of peaks and ebbs in the flow of nutrients and secondary metabolites, endocrine signals of the fed/fasting state, and neural outputs may thus interact to imprint the liver clock to cycles of feeding behavior established by the SCN.
Circadian Dysregulation Is Both Symptomatic and Causative of Metabolic Disease
The first experiments demonstrating the significance of clock genes to metabolic homeostasis involved mice with mutations in the Clock gene (22). Mice homozygous for mutations in the Clock gene exhibited obesity in chow-fed conditions, fasting hyperglycemia, elevated lipids and hepatic steatosis, and increased propensity for diet-induced obesity (DIO) when fed refined diets with high content of saturated fats and sucrose. The phenotype was attributed to hyperphagia during periods normally associated with sleep. The same group reported soon thereafter that feeding C57BL/6J mice a high-fat/high-sucrose diet frequently used to induce obesity rapidly comprised circadian rhythms and the normal diurnal profiles in feeding behavior and gene expression (23).
Experiments using conditional gene targeting indicate that core elements of the molecular clock expressed in the liver are essential per se for normal rhythms in liver function (24-28). For example, deletion of the Bmal1 gene in the liver results in hypoglycemia and reduced hepatic glucose production (28). As discussed later in this review, activation of lipogenesis observed in the fatty liver of obese mice also appears to have a circadian component that is independent of the canonical clock (29-31). Dysregulation of the liver clock, and possibly the cues from oscillators active outside the liver, may thus contribute to the pathogenesis of liver disease. Accordingly, components of the molecular clock are now being actively investigated as drug targets (32).
These early experiments have been translated to several observations in human physiology and pathology. Studies using human volunteers have demonstrated that desynchronizing circadian rhythms results in metabolic dysregulation and elevated risk of adverse cardiometabolic outcomes (33-36). On the flip side, restricting caloric intake to be in alignment (timed restricted feeding, TRF) improves cardiometabolic health in humans (4, 37, 38). Whether this approach benefits NAFLD has yet to be reported, although clinical trials are in progress (eg, ClinicalTrials.gov identifier: NCT03786523). TRF has however been reported to prevent metabolic dysregulation and a fatty liver phenotype in C57BL/6J mice fed a high-fat diet (39).
Liver Hepatocyte as a “Relay Station” in the Diurnal Rhythm of Nutrient Trafficking
Hepatocytes are the most abundant specialized cell type of the liver and house the enzymatic machinery that is involved in the homeostatic responses to variability in nutrient intake. Following a meal, the end products of nutrient digestion are delivered from the small intestine to the liver via the portal vein. Hepatocytes convert some of the ingested glucose to glycogen for storage within the liver. The liver also converts excess carbohydrates into fatty acids (FAs), which can then be stored for future use as triacylglycerol (TAG). Hepatocytes are also a major site of de novo cholesterol synthesis, using acetyl coenzyme A derived from oxidation of glucose and FAs. In healthy individuals, the liver does not store large amounts of lipids. Triacylglycerol, cholesterol, and cholesterol ester are packaged into very low-density lipoproteins (VLDLs) and secreted into the circulatory system. The lipid cargo of VLDL is then released to other specialized cell types throughout the body to be used as fuel for adenosine 5′-triposphate production, as substrates for cellular homeostasis, or stored as lipid droplets.
The liver also orchestrates the metabolic adaptation to fasting and nutrient scarcity. Glucose is maintained between normal physiological levels (euglycemia) by activating glycogenolysis and secreting glucose extracted from glycogen back into the circulation. The liver can also produce glucose by activating gluconeogenesis using noncarbohydrate substrates (lactate, glycerol, alanine, and glutamine). In addition, the liver converts FAs released from adipocytes and ketogenic amino acid to ketones (ketogenesis). These processes provide substrates for energy production to the highly specialized cell populations found in the nervous system, cardiovascular system, and skeletal muscle in situations of prolonged nutrient scarcity.
An Interplay Between Hepatic and Extrahepatic Clocks
The metabolic functions required of hepatocytes to maintain homeostasis normally follow a diurnal rhythm driven by the combined actions of “local” and systemic oscillators (see Fig. 2). Recent experiments suggest that although the liver clock can operate independently of other oscillators, the full expression of normal rhythms is dependent on oscillators outside the liver. To investigate the functions of cell-autonomous circadian oscillators in specific tissues, investigators have used the “Cre-Lox” system. Gene transcription can be manipulated by inserting a “transcriptional block” flanked by LoxP sites in the 5′ untranslated region of a gene. The presence of the foreign DNA in the gene silences expression, resulting in a global knockout. The expression of the bacteriophage Cre recombinase results in the removal of this sequence, restoring normal expression. “Complete” Bmal1 knockout mice that are unable to express the BMAL1 protein in any tissue are unable to maintain a circadian rhythm in constant darkness and display an array of pathologies indicating an accelerated aging phenotype (growth retardation, loss of bone [osteoporosis] and muscle [sarcopenia], and cataracts) (9, 40). Experiments in which the transcriptional block inserted into the Bmal1 gene is removed in the liver using a transgenic line expressing hepatocyte-specific Cre transgene indicate that the liver clock can maintain a rhythm in the absence of other circadian oscillators in the body (41). In the absence of other body clocks, the liver clock expresses a rhythm with advanced timing. However, there is a 90% reduction in the number of genes exhibiting rhythms. The liver clock appears to be sufficient for circadian rhythms in glycogen and nicotinamide adenine dinucleotide (NAD+) metabolism. Rhythms in the expression of genes involved in lipid and xenobiotic metabolism are not restored, suggesting a requirement for signals from other clocks. The results of this experiment also indicate that liver secretions driven by hepatocyte-autonomous clocks are insufficient to drive normal rhythms of feeding behavior and associated metabolic cycles in fuel selection and energy expenditure.
Candidates for external cues that regulate hepatic metabolism include autonomic and endocrine signals (see Fig. 2). The release of hormones from the pituitary gland exhibits a circadian profile (42). The list of hormones released from the pituitary that influence metabolism includes corticotropin-releasing hormone, growth hormone (GH), thyroid hormone, and gonadal steroids. The release of these hormones is known to be affected by nutrient intake, and all have important effects on liver function.
Differences in the pattern of GH secretion from the pituitary underlie sex differences in the hepatic gene expression profile, particularly in rodents (43). GH secretion also exhibits a circadian profile, peaking at nighttime in humans. Restricted feeding results in an altered circadian profile in GH secretion (44). The release of thyrotropin, which stimulates the secretion of thyroxine from the thyroid, also exhibits a circadian profile with a nighttime peak in humans (45, 46). Activation of the primary isoform of the thyroid hormone expressed in the liver (THRβ), a ligand-dependent nuclear receptor that functions as a TF, regulates FA and cholesterol metabolism (47). As for GH, studies in rodents indicate altered feeding patterns modify thyroid hormone secretions in rats (48, 49). Although it seems likely that GH and thyroid hormones contribute to the diurnal profile in hepatic gene expression, their contribution to the response of the liver clock to phase shifts in nutrient intake remain unclear.
Corticotropin-releasing hormone is a component of the hypothalamic-pituitary-adrenal axis, stimulating the synthesis and secretion of glucocorticoids (cortisol in most mammals, corticosterone in laboratory rodents) from the adrenal gland. Glucocorticoids are well known to be involved in metabolic adaptation to nutrient scarcity, a role that includes activating glucocorticoid receptors in the liver to increase expression of genes involved in hepatic gluconeogenesis (50, 51). Administration of dexamethasone induces phase shifts in Per1 expression in fibroblasts and mouse liver (52). However, glucocorticoids appear to have an inhibitory effect on the phase shifts in the liver clock when feeding time is altered (53, 54).
Endocrine signals of the fed/fasted condition are also delivered to the liver from the islets of Langerhans in the pancreas via the portal vein. The release of insulin from β cells after a meal acts to regulate the fate of ingested carbohydrates and lipids, and also controls the release of glucose from the liver. Insulin acts rapidly to suppress glycogenolysis, increase glycogen synthesis, and activates programs to shunt excess glucose into de novo lipogenesis.
Insulin secretion is regulated by cell-autonomous circadian oscillators. Mice lacking functional Bmal1 or Clock genes exhibit reduced islet mass and are hypoinsulinemic, resulting in impaired glucose tolerance. (55). Genes encoding proteins involved in insulin secretion exhibit a circadian profile in β cells, while deletion of core clock genes results in a deficit in the expression of proteins involved in exocytosis and nutrient-dependent stimulation of insulin secretion (56, 57). Collectively, these experiments suggest that the impact of nutrient intake on insulin secretion by β cells is modulated by the clock being synchronized with meal timing. In support of the clinical relevance of these findings, isolated human islets exhibit evidence for cell-autonomous clocks regulating insulin secretion that is severely attenuated in patients with type 2 diabetes (58).
Our understanding of the role of glucagon released from pancreatic α cells, and of the interplay between insulin and glucagon, in regulating hepatic glucose and lipid metabolism is complex and evolving (59, 60). However, glucagon secretion in isolated human islets also exhibits a circadian profile that is disrupted in type 2 diabetes (58). Moreover, the clock driving hormone secretion in α cells appears to be about 8 hours out of phase with the clocks in β cells (61). Glucagon could be an endocrine signal to the liver of the fed/fasted state. Activation of the glucagon receptor in the “intermeal interval” appears to contribute to the response of the liver clock to phase shifts in nutrient availability (54, 62).
Modeling of interactions between insulin and glucagon in the control hepatic glucose and lipid metabolism has historically focused on responses to glucose (59, 60). The secretion of glucagon is inhibited by glucose, while glucagon activates hepatic glycogenolysis and gluconeogenesis. This led to the hypothesis that glucagon acts as a counterregulatory hormone during hypoglycemia. However, the responses of insulin and glucagon are not indicative of what happens following the ingestion of a mixed meal. Whereas glucagon secretion is inhibited by glucose infusions, a mixed meal actually has the opposite effect and increases glucagon secretion. Clearly, significant gaps in knowledge still exist in our understanding of the role played by glucagon in metabolic homeostasis. Indeed, the primary functions of glucagon in the liver may extend beyond the control of glucose homeostasis to include hepatic amino acid metabolism (59, 63).
Obese insulin-resistant mice exhibit hepatic steatosis and increased expression of lipogenic enzymes in the liver, mediated at least in part by increased activation of insulin-responsive sterol regulatory element binding proteins (SREBPs) (64, 65). The initial results from experiments examining how overnutrition and obesity affect the liver clock indicated an inhibitory response (23). However, a more recent analysis of the liver transcriptome in DIO mice indicates a more complicated interpretation. Obesity results in subtle changes in rhythms of core clock genes (Bmal1, Per2, Rev-erbα). However, there appears to be a marked inhibition of the recruitment of BMAL1 and CLOCK to the promoters of known target genes (31). In addition, a large group of genes actually attained rhythmicity in mice fed a high-fat diet driven primarily by peroxisome proliferator-activated receptor γ (PPARγ), a nuclear receptor that regulates glucose and lipid metabolism, but also involved SREBP1 (31). PPARγ deletion has been reported to reduce rhythmicity in the expression of core clock genes and clock target genes in chow-fed mice (66), whereas the PPARγ agonist rosiglitazone reverses the impact of DIO and diabetes on rhythmicity in hepatic gene expression (67, 68). A study by another group also reported that DIO induced rhythmicity in SREBP1 and genes involved in lipid synthesis (29). However, the authors of this study suggested that increased SREBP1 activation of gene transcription of lipogenic enzymes results in the synthesis of a ligand of PPARα, a nuclear receptor involved in FA oxidation and the liver fasting response.
The reasons for the different outcomes observed by the 2 laboratories discussed here are not clear, but likely include variability in the housing environment at each facility. One interpretation is that multiple pathways can lead to activation of rhythmic gene expression in the liver. Indeed, it is also worth noting that a similar phenotype has been reported in a genetic model of mild obesity. Mice lacking melanocortin-3 receptors (MC3Rs) develop a modest obese phenotype that appears to be primarily metabolic in nature (69, 70). Mc3r–/– mice subjected to a TRF protocol using a low-fat, high-sugar diet that also limits calories to induce negative energy balance and weight loss exhibit marked deficits in rhythmicity of liver Bmal1 and Rev-erbα expression (30). However, TRF induced a marked rhythmicity in liver SREBP1 and PPARγ expression that anticipated food presentation. In this study, the anticipatory peaks in SREBP1 correlated with serum insulin levels, which also increased pre meal. These findings suggest that similar phenotypes may occur in genetic models of hypothalamic obesity.
Neuronal populations in the hypothalamus have been proposed to integrate signals of photoperiod and metabolic state, relaying this information to peripheral organ system via the autonomic nervous system (71, 72). Targeted deletion of Bmal1 in the forebrain and SCN interferes with the expression of diurnal rhythms of activity, feeding behavior, and fuel selection (glucose vs fat oxidation) (73). These mice also exhibit impaired glucose tolerance, increased hepatic glucose production, and dysregulated rhythmicity in the hepatic expression of genes involved in carbohydrate and lipid metabolism. Specific deletion of Bmal1 in agouti-related peptide (AgRP) neurons results in a similar phenotype (73). AgRP neurons are a focal point in the hypothalamic responses to signals of energy balance received from peripheral tissues. These results indicate that the activity of cell-autonomous clocks in hypothalamic neurons is relevant to liver function.
Timed Restricted Feeding Restores Rhythmicity in the Liver Clock
Because the liver clock is both directly and indirectly regulated by feeding/fasting and overnutrition often involves dysregulated meal timing, it has been postulated that TRF may improve the liver clock and hepatic metabolic cycles. An initial report in C57BL/6J mice described that 8-hour TRF reduced body weight despite unaltered total caloric intake and had minor effects on hepatic clock gene expression of chow-fed mice but dramatically increased the amplitude of clock gene rhythmicity in high-fat–fed mice (39). This TRF paradigm rescued or enhanced the gene expression rhythms of hepatic genes for glucose and lipid metabolism, and restored hepatic concentrations of several glycolytic, lipid, and tricarboxylic acid cycle metabolites. Lastly, this study also reported complete correction of the DIO-induced hepatic steatosis. A second group simultaneously reported that a 4-hour TRF paradigm during the light phase reduced body weight with some reduction in caloric intake, induced a shift in hepatic clock gene expression rhythms, and despite enhancing liver fat oxidation gene expression did not decrease the high-fat diet–induced hepatic steatosis (74). The first group also reported in a follow-up study that 8- to 9-hour TRF when fed either a high fat and sucrose, or 60% high-fat diet was able to reduce hepatic triglycerides whether the TRF was applied during the duration of the study, for a shorter amount of time after ad libitum feeding and development of obesity, or even if the TRF was stopped and 2 weeks of ad libitum feeding was allowed (75).
TRF in all these scenarios altered either the amplitude or timing relative to light/dark cycles of several lipid and glucose metabolic gene expression oscillations. More recently, this group studied mice lacking the core hepatic clock genes: either Cry1/Cry2 whole-body knockout mice, or liver-specific Bmal1 or Rev-erbα/β knockout mice. Nine to 10 hours TRF of high-fat diet feeding in these hepatic clock–deficient mice was able to reduce hepatic triglycerides and was associated with a more normalized pattern of the liver transcriptome and metabolome, despite the absence of a liver clock (76). Interestingly, another model of clock-deficient mice (Per1/2 whole-body knockout) also maintained some hepatic fatty acid/triglyceride gene expression as well as some lipid and triglyceride level oscillations (77). Altogether, these results suggest that the liver’s circadian rhythm is regulated primarily by feeding/fasting cycles and is not dependent on its own core clock genes. Thus, sustaining periods of feeding and fasting helps the liver to maintain metabolic oscillations despite consumption of high-fat and high-carbohydrate diets.
In addition to obesity-related NAFLD, hepatic circadian rhythms have been described to be altered with aging of mice (78). This study demonstrated reduced circadian expression of the core hepatic clock genes, altered levels of NAD+ metabolites, and decreased circadian rhythm of protein acetylation posttranslational modifications in aged livers. In this study, caloric restriction likely mimicked TRF in this study, which involved a 30% reduction in calories compared to ad libitum with food provided only once a day at zeitgeber time 12. This experiment was able to enhance the circadian expression of core hepatic clock genes, increase the number of cyclical gene transcripts, and increase protein acetylation throughout the proteome, including on circadian gene promoter regions. Thus, it appears that resynchronizing the hepatic clock with timed caloric restriction can correct the NAD+-related metabolic deficits observed with aging. Whether the same outcome would be observed with TRF not involving limited calories needs to be determined.
Conclusions and Areas of Future Investigation
The liver responds to food intake by helping to regulate carbohydrate and lipid storage or trafficking to peripheral tissues. Likewise, hepatic metabolism is important for nutritional homeostasis during fasting periods by producing glucose from glycogen stores or gluconeogenesis from noncarbohydrate sources, as well as producing ketone bodies from lipid and ketogenic amino acid oxidation. Gene expression related to these metabolic pathways predominantly displays rhythmic patterns, which are related to hepatic clock–regulating genes, the master hypothalamic/pituitary clock, as well as direct and indirect nutrient signals. Recent studies in rodents suggest that feeding/fasting are the strongest regulators of these hepatic oscillations. Overnutrition often involves dysregulated feeding cycles and can either dampen or disorder these hepatic metabolic oscillations.
In rodents, TRF of normal or high-calorie diets allows for maintained hepatic rhythms in glucose and lipid metabolism, even in the absence of core hepatic clock genes. These studies primarily suggest improved hepatic lipid accumulation, which may be related to this maintained metabolic gene expression, or also to the reduced obesity and insulin resistance that is observed with TRF. There is little information as to whether the hepatic clock or TRF is important in NASH pathology. A synthetic agonist of Rev-erbα/β, which could inhibit not only clock gene Bmal1 expression but also lipid metabolic gene expression (see Fig. 1), was recently described to improve inflammation and fibrosis, but surprisingly did not improve steatosis in a mouse model of NASH (79). Many other NASH therapeutics in development also target nuclear receptors that interplay with the circadian clock.
Another outstanding question remains the importance of the clock or TRF in human hepatic physiology or pathology. And if TRF is a therapeutic strategy for NAFLD/NASH in humans, are the improvements due to resynchronization of the hepatic clock, or improved obesity and metabolic function? However, unlike the weight loss observed in laboratory rodents, TRF may not induce weight loss in humans (80). Thus, a primary goal for this area of research will be to identify whether TRF is a beneficial therapeutic intervention for NAFLD/NASH, and whether its effects are related directly to the hepatic clock, or to reductions in caloric intake, obesity, and metabolic dysfunction.
Acknowledgments
Financial Support: This work is supported by the National Heart, Lung, and Blood Institute (grant No. R00 HL136658 to K.S.M.) and the National Institute of Neurological Disorders and Stroke (grant No. R21 NS108138 to A.A.B.).
Glossary
Abbreviations
- AgRP
agouti-related peptide
- BMAL1
brain or muscle ARNT-like 1
- CLOCK
circadian locomotor output cycles kaput
- Cry
cryptochrome
- DIO
diet-induced obesity
- FA
fatty acid
- GH
growth hormone
- MC3R
melanocortin-3 receptor
- NAD+
nicotinamide adenine dinucleotide
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NPAS2
neuronal PAS domain protein 2
- PAS
Per-Arnt-Sim
- Per
period
- PPARγ
peroxisome proliferator-activated receptor γ
- ROR
retinoic acid–related orphan receptor
- SCN
suprachiasmatic nucleus of the hypothalamus
- SREBP
sterol regulatory element binding protein
- TAG
triacylglycerol
- TF
transcription factor
- TRF
timed restricted feeding
- VLDL
very low-density lipoprotein
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the writing of this review article.
References
- 1. Afshin A, Forouzanfar MH, Reitsma MB, et al. ; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1): 13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younossi ZM, Tampi RP, Racila A, et al. Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the U.S. Diabetes Care. 2020;43(2): 283-289. [DOI] [PubMed] [Google Scholar]
- 3. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11-20. [DOI] [PubMed] [Google Scholar]
- 4. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6): 1212-1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring). 2019;27(8):1244-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hutchison AT, Regmi P, Manoogian ENC, et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27(5):724-732. [DOI] [PubMed] [Google Scholar]
- 8. Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994-999. [DOI] [PubMed] [Google Scholar]
- 9. Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10(5):543-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shostak A, Brunner M. Help from my friends—cooperation of BMAL1 with noncircadian transcription factors. Genes Dev. 2019;33(5-6):255-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho H, Zhao X, Hatori M, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bugge A, Feng D, Everett LJ, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26(7):657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato TK, Panda S, Miraglia LJ, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43(4):527-537. [DOI] [PubMed] [Google Scholar]
- 15. Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6381):eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anafi RC, Francey LJ, Hogenesch JB, Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A. 2017;114(20):5312-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490-493. [DOI] [PubMed] [Google Scholar]
- 19. Tahara Y, Kuroda H, Saito K, et al. In vivo monitoring of peripheral circadian clocks in the mouse. Curr Biol. 2012;22(11):1029-1034. [DOI] [PubMed] [Google Scholar]
- 20. Wehrens SMT, Christou S, Isherwood C, et al. Meal timing regulates the human circadian system. Curr Biol. 2017;27(12):1768-1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13(3):197-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414-421. [DOI] [PubMed] [Google Scholar]
- 24. Jacobi D, Liu S, Burkewitz K, et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015;22(4):709-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan X, Bradfield CA, Hussain MM. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat Commun. 2016;7:13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma D, Liu T, Chang L, et al. The liver clock controls cholesterol homeostasis through Trib1 protein-mediated regulation of PCSK9/low density lipoprotein receptor (LDLR) axis. J Biol Chem. 2015;290(52):31003-31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang D, Tong X, Arthurs B, et al. Liver clock protein BMAL1 promotes de novo lipogenesis through insulin-mTORC2-AKT signaling. J Biol Chem. 2014;289(37):25925-25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105(39):15172-15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guan D, Xiong Y, Borck PC, et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell. 2018;174(4):831-842.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sutton GM, Begriche K, Kumar KG, et al. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010;24(3):862-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eckel-Mahan KL, Patel VR, de Mateo S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155(7):1464-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Z, Yoo SH, Takahashi JS. Development and therapeutic potential of small-molecule modulators of circadian systems. Annu Rev Pharmacol Toxicol. 2018;58:231-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qian J, Morris CJ, Caputo R, Wang W, Garaulet M, Scheer FAJL. Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci U S A. 2019;116(47):23806-23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wefers J, van Moorsel D, Hansen J, et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci U S A. 2018;115(30):7789-7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402-E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20(14):1868-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koronowski KB, Kinouchi K, Welz PS, et al. Defining the independence of the liver circadian clock. Cell. 2019;177(6):1448-1462.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol. 2014;10(8):466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20(11):2613-2629. [DOI] [PubMed] [Google Scholar]
- 44. Arellanes-Licea Edel C, Báez-Ruiz A, Carranza ME, Arámburo C, Luna M, Díaz-Muñoz M. Daily patterns and adaptation of the ghrelin, growth hormone and insulin-like growth factor-1 system under daytime food synchronisation in rats. J Neuroendocrinol. 2014;26(5):282-295. [DOI] [PubMed] [Google Scholar]
- 45. Allan JS, Czeisler CA. Persistence of the circadian thyrotropin rhythm under constant conditions and after light-induced shifts of circadian phase. J Clin Endocrinol Metab. 1994;79(2):508-512. [DOI] [PubMed] [Google Scholar]
- 46. Depner CM, Melanson EL, McHill AW, Wright KP Jr. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc Natl Acad Sci U S A. 2018;115(23):E5390-E5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14(5):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ortiz-Caro J, González C, Jolin T. Diurnal variations of plasma growth hormone, thyrotropin, thyroxine, and triiodothyronine in streptozotocin-diabetic and food-restricted rats. Endocrinology. 1984;115(6):2227-2232. [DOI] [PubMed] [Google Scholar]
- 49. Cokelaere M, Decuypere E, Flo G, Darras VM, Kühn ER. Influence of feeding pattern on thyroid hormones in long-term food-restricted rats. Horm Metab Res. 1996;28(7):315-318. [DOI] [PubMed] [Google Scholar]
- 50. Bose SK, Hutson I, Harris CA. Hepatic glucocorticoid receptor plays a greater role than adipose GR in metabolic syndrome despite renal compensation. Endocrinology. 2016;157(12):4943-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Opherk C, Tronche F, Kellendonk C, et al. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol. 2004;18(6): 1346-1353. [DOI] [PubMed] [Google Scholar]
- 52. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344-2347. [DOI] [PubMed] [Google Scholar]
- 53. Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20(24):7128-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mukherji A, Kobiita A, Chambon P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Proc Natl Acad Sci U S A. 2015;112(48):E6683-E6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perelis M, Marcheva B, Ramsey KM, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350(6261):aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marcheva B, Perelis M, Weidemann BJ, et al. A role for alternative splicing in circadian control of exocytosis and glucose homeostasis. Genes Dev. 2020;34(15-16):1089-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Petrenko V, Gandasi NR, Sage D, Tengholm A, Barg S, Dibner C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc Natl Acad Sci U S A. 2020;117(5): 2484-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. El K, Capozzi ME, Campbell JE. Repositioning the alpha cell in postprandial metabolism. Endocrinology. 2020;161(11):bqaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Finan B, Capozzi ME, Campbell JE. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes. 2020;69(4):532-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Petrenko V, Saini C, Giovannoni L, et al. Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev. 2017;31(4):383-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun X, Dang F, Zhang D, et al. Glucagon-CREB/CRTC2 signaling cascade regulates hepatic BMAL1 protein. J Biol Chem. 2015;290(4):2189-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, et al. The liver-α-cell axis and type 2 diabetes. Endocr Rev. 2019;40(5):1353-1366. [DOI] [PubMed] [Google Scholar]
- 64. Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6(1): 77-86. [PubMed] [Google Scholar]
- 65. Albarado DC, McClaine J, Stephens JM, et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145(1):243-252. [DOI] [PubMed] [Google Scholar]
- 66. Yang G, Jia Z, Aoyagi T, McClain D, Mortensen RM, Yang T. Systemic PPARγ deletion impairs circadian rhythms of behavior and metabolism. PLoS One. 2012;7(8):e38117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tseng HL, Yang SC, Yang SH, Shieh KR. Hepatic circadian-clock system altered by insulin resistance, diabetes and insulin sensitizer in mice. PLoS One. 2015;10(3):e0120380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ribas-Latre A, Fekry B, Kwok C, et al. Rosiglitazone reverses high fat diet-induced changes in BMAL1 function in muscle, fat, and liver tissue in mice. Int J Obes (Lond). 2019;43(3): 567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Butler AA, Kesterson RA, Khong K, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518-3521. [DOI] [PubMed] [Google Scholar]
- 70. Chen AS, Marsh DJ, Trumbauer ME, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26(1):97-102. [DOI] [PubMed] [Google Scholar]
- 71. Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161(1):133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cedernaes J, Waldeck N, Bass J. Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Genes Dev. 2019;33(17-18):1136-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cedernaes J, Huang W, Ramsey KM, et al. Transcriptional basis for rhythmic control of hunger and metabolism within the AgRP neuron. Cell Metab. 2019;29(5):1078-1091.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26(8):3493-3502. [DOI] [PubMed] [Google Scholar]
- 75. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29(2):303-319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Adamovich Y, Rousso-Noori L, Zwighaft Z, et al. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19(2):319-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sato S, Solanas G, Peixoto FO, et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170(4):664-677.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Griffett K, Bedia-Diaz G, Elgendy B, Burris TP. REV-ERB agonism improves liver pathology in a mouse model of NASH. PLoS One. 2020;15(10):e0236000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lowe DA, Wu N, Rohdin-Bibby L, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the writing of this review article.