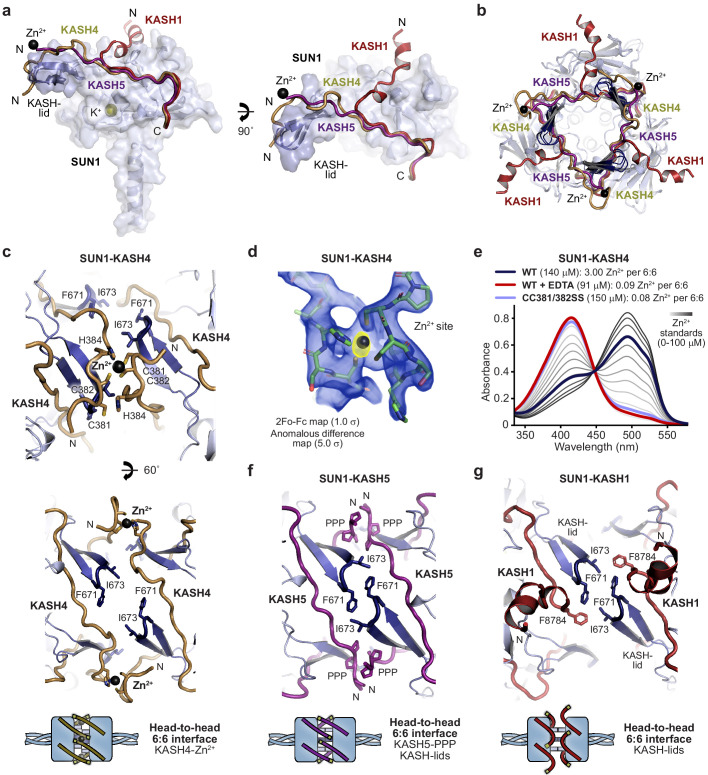
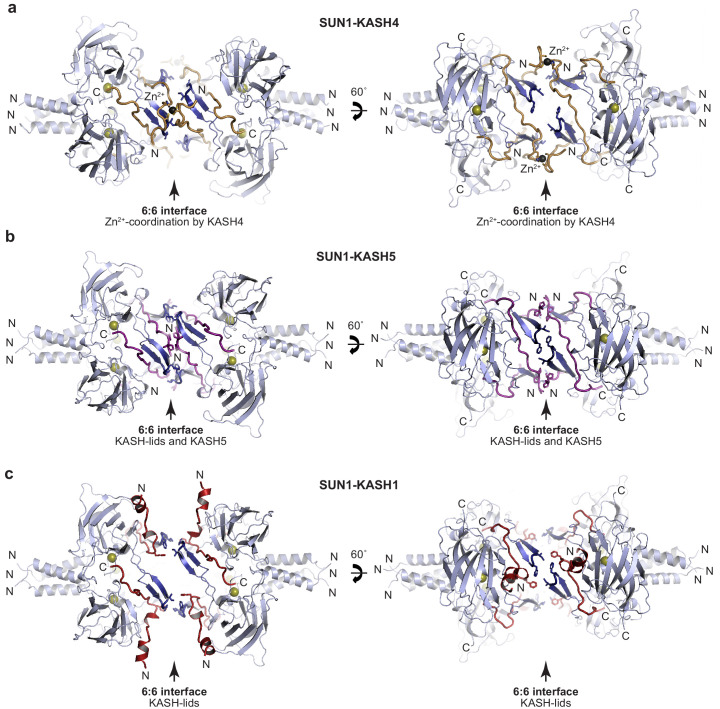
Figure 3. Specialised KASH sequences provide distinct SUN-KASH 6:6 assembly mechanisms.
(a) SUN-KASH 1:1 protomers from SUN1-KASH4, SUN1-KASH5, and SUN1-KASH1 crystal structures, superposed, and displayed as the SUN1 molecular surface with KASH-lids highlighted in blue as cartoons, and KASH sequences represented as cartoons (yellow, purple, and red, respectively). (b) Cross-section through the head-to-head interface of superposed SUN1-KASH4, SUN1-KASH5, and SUN1-KASH1 6:6 assemblies such that their constituent 3:3 complexes are visible. (c) Structural details of the SUN1-KASH4 6:6 interface, showing a zinc-binding site in which opposing KASH4 chains provide asymmetric ligands C381 and C382, and C382 and H384 (top), and the lack of interface-spanning interactions between opposing SUN1 KASH-lids (bottom). (d) 2Fo-Fc (blue) and anomalous difference (yellow) electron density maps contoured at 1.0 σ and 5.0 σ, respectively, at a zinc-binding site of SUN1-KASH4. (e) Spectrophotometric determination of zinc content for SUN1-KASH4 wild-type (dark blue; 3.00 Zn2+ per 6:6), wild-type with EDTA treatment prior to gel filtration (red; 0.09 Zn2+ per 6:6), and CC381/382SS (light blue; 0.08 Zn2+ per 6:6), using metallochromic indicator PAR, with zinc standards shown in a gradient from light to dark grey (0–100 µM). Representative of three replicates. (f) Structural details of the SUN1-KASH5 6:6 interface, demonstrating interface-spanning interactions between PPP-motifs (amino acids 545-PPP-547) of opposing KASH5 chains, and between amino acids F671 and I673 of opposing SUN1 KASH-lids. (g) Structural details of the SUN1-KASH1 6:6 interface showing interactions between amino acids F671 and I673 of opposing SUN1 KASH-lids that are supported by KASH1 amino acid F8784, but with no interface-spanning interactions between opposing KASH1 chains.
Figure 3—figure supplement 1. SUN-KASH crystal structures.
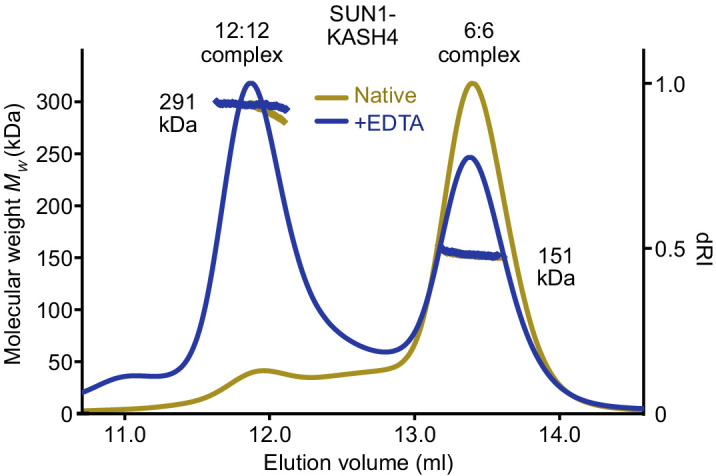
Figure 3—figure supplement 2. SUN1-KASH4 forms 6:6 and 12:12 complexes upon sequestration of bound zinc SEC-MALS analysis of SUN1-KASH4 (yellow) and following the removal of bound zinc (demonstrated in Figure 3e) by treatment with EDTA (blue).