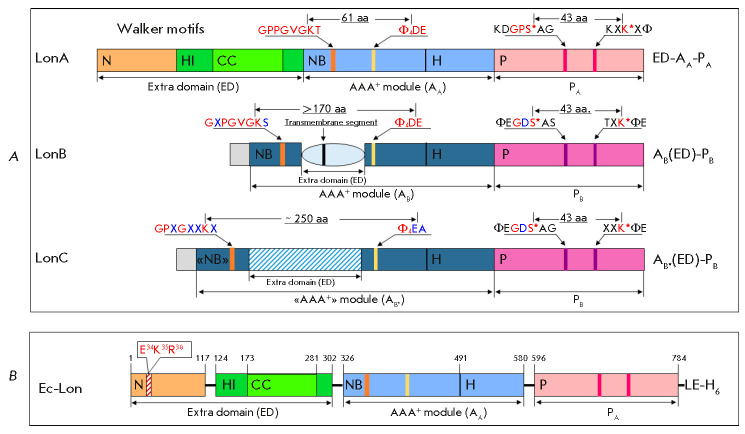
Fig. 1.
Domain organization of Lon proteases from different subfamilies (A) and domain boundaries in the subunit of E. coli Lon protease (B). (A) S* and K* – catalytic residues of the proteolytic active site; Φ – hydrophobic amino acid residue; X – any amino acid residue; PA and PB – A-type (pink) and B-type (purple) protease domains; AA, AB, and AB* – AAA+ modules of A-type (light blue), B-type (blue), and “degenerate” B*-type (blue), respectively; NB – nucleotide-binding domain; H – α-helical domain; ED – extra domains represented by the N-domain (brown) and inserted α-helical HI(CC) domain (green) with a coiled-coil region (light green) in LonA proteases, a transmembrane domain (light blue) in LonB, and an inserted domain (shaded) in LonC; aa – amino acid residue; amino acid substitutions in conserved fragments are highlighted in blue. (B) E. coli Lon protease subunit with a C-terminal 6His-tag; the N domain region comprising E34, K35, and R38 residues is shaded