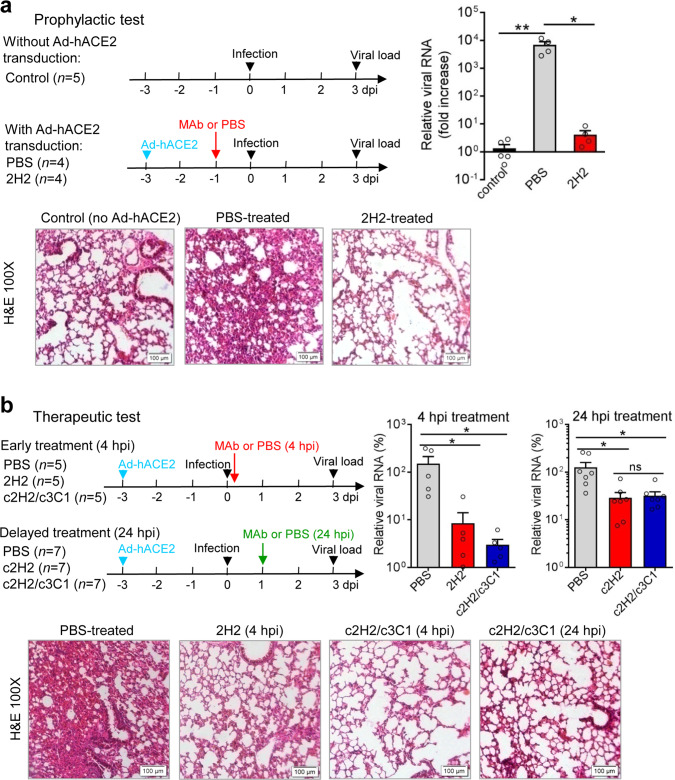
Fig. 3. Protective efficacy of MAb 2H2 and the chimeric antibody cocktail against authentic SARS-CoV-2 infection in mice.
a, b In vivo prophylactic efficacy (a) and therapeutic efficacy (b) of MAb 2H2, c2H2, and/or the c2H2/c3C1 cocktail against SARS-CoV-2 infection. Upper left panel: study outline. Upper right panel: qRT-PCR analysis of viral RNA copies present in lung tissues after 3 days of infection. Lower panel: H&E staining of lung tissue sections at 3 d.p.i. For a, qPCR results are shown as fold increase relative to wide-type Balb/c group (without Ad5-hACE2 treatment). For b, qPCR results are expressed as viral RNA levels in different antibody treatment groups relative to that in the PBS control group. For top right panels in a and b, each symbol represents one mouse. Error bars represent SEM. Statistical significance was determined by a two-tailed Student’s t test and indicated as follows: ns not significant; *p < 0.05; **p < 0.01. For a, p value between the control group and the PBS group (Ad-hACE2 transduction) is 0.0073; p value between the PBS group and the 2H2 group is 0.0169. For early treatment experiment in b, p value between the PBS group and the 2H2 group is 0.0488; p value between the PBS group and the c2H2/c3C1 group is 0.0418. For delayed treatment experiment in b, p value between the PBS group and the c2H2 group is 0.0183; p value between the PBS group and the c2H2/c3C1 group is 0.0205; p value between the c2H2 group and the c2H2/c3C1 group is 0.7803.