Abstract
Air pollution is a worrisome risk factor for global morbidity and mortality and plays a special role in many respiratory conditions. It contributes to around 8 million deaths/year, with outdoor exposure being responsible for more than 4.2 million deaths throughout the world, while more than 3.8 million die from situations related to indoor pollution. Pollutant agents induce several respiratory symptoms. In addition, there is a clear interference in numerous asthma outcomes, such as incidence, prevalence, hospital admission, visits to emergency departments, mortality, and asthma attacks, among others. The particulate matter group of pollutants includes coarse particles/PM10, fine particles/PM2.5, and ultrafine particles/PM0.1. The gaseous components include ground-level ozone, nitrogen dioxide, sulfur dioxide, and carbon monoxide. The timing, load, and route of allergen exposure are other items affecting allergic disease phenotypes. The complex interaction between pollutant exposures and human host factors has an implication in the development and rise of asthma as a public health problem. However, there are hiatuses in the understanding of the pathways in this disease. The routes through which pollutants induce asthma are multiple, and include the epigenetic changes that occur in the respiratory tract microbiome, oxidative stress, and immune dysregulation. In addition, the expansion of the modern Westernized lifestyle, which is characterized by intense urbanization and more time spent indoors, resulted in greater exposure to polluted air. Another point to consider is the different role of the environment according to age groups. Children growing up in economically disadvantaged neighborhoods suffer more important negative health impacts. This narrative review highlights the principal polluting agents, their sources of emission, epidemiological findings, and mechanistic evidence that links environmental exposures to asthma.
Keywords: Air pollution, Particulate matter, Asthma
Introduction
Air pollution is currently one of the most significant preventable health risks worldwide. It has been called a “silent killer” by the World Health Organization (WHO) [1] because its effects often go unnoticed and are not easily measured, nor it is associated with health problems by the lay population [2]. However, air pollution is a major cause of premature death [3–7]. Another WHO recent alert is about that 90% of the world’s population breathes inadequate air—a fact that has not had the expected repercussions [8]. Air pollution is responsible for approximately 4.2 million deaths in 2015. Air pollution also caused over 100 million years of life lost, adjusted to the number of years lived with disability (DALYs) [4]. The European Environmental Agency (EEA) reported that exposure to particulate matter and gaseous pollutants above the recommended levels still remains very common in many countries [2, 5, 9].
External environmental pollution agents are dispersed by wind currents and do not obey country borders. For example, polluting agents related to Chinese industrial production reach Japan and the west coast of the USA. These pollutants are driven by wind currents [5]. A similar situation takes place with the smoke resulting from wildfires in the Amazon rainforest; the air pollutants from these clouds disperse over long distances [10]. However, the impact of these burns on mortality and morbidity from acute and chronic respiratory and cardiovascular outcomes requires more reliable indicators. Using satellite technology, National Aeronautics and Space Administration (NASA)-supported scientists are developing markers to evaluate global air pollution and the role of the main pollutant agents in some respiratory diseases, such as asthma. Researchers have already incorporated some of these indicators in tracking pollution clouds from wildfires, dust storms, pollens, urban green space, tropospheric ozone concentrations, particulate matter, and many others [11, 12].
An evidence accumulated for several decades supports the idea that air pollution can exacerbate pre-existing asthma. However, new findings have emerged that suggest air pollution might also cause new-onset asthma.
There is a relationship between the production of air pollutants with some habits of the population in certain regions or countries and with regard to specific professional situations. An example are the workers’ migration from workplaces like fields and farms to factories and offices. Urbanization and economic growth in many countries are responsible for the increase in some industrial activities and for the significant increase in motor vehicle emissions. These factors caused severe air pollution. The westernization of the way of life of a large part of the world’s population, with consequent changes in customs, led to the voluntary isolation of families, whose members stay longer in their homes or work environments, thereby increasing the impact of indoor pollution [13–15]. It is accepted that about 80–90% of the time, urban families stay in indoor environments, which reinforces the importance of air quality in these environments [16–18]. In some societies, people spend a large amount of time indoors with negative consequences on their health and well-being. Poor indoor air quality can trigger asthma symptoms, likely related to exposure to common triggers such as household dust, mold, and tobacco smoke. Household air quality can be 2 to 5 times worse than outdoor air quality, and unlike the latter, there are no laws to regulate it. Indoor air pollution is determined partly by outdoor air quality depending on ventilation systems and cleaning practices in residences [5, 13].
There is an interplay of indoor and outdoor environmental exposures interacting with host factors. The response to different exposures depends on the individual's genetic characteristics. This complex interaction is associated with the development and progression of allergic diseases, but the timing, load, and route of allergen exposure also have an important effect on allergic disease progression and the severity of symptoms.
Chemical pollution is another form of aggression to the environment and health. According to Landrigan et al., industries synthesized more than 140,000 new chemical agents and pesticides in recent years, with significant spread across the planet, and without assessment of possible deleterious effects before large-scale use [5]. Chemical exposures associated with occupational asthma, especially in atopic individuals, include pharmaceuticals, cosmetic products, flame-retardants, and many others.
There is no reliable control of the several forms of pollution related to industries, agriculture, and the burning of fossil fuels by motor vehicles, in both developed and newly industrialized countries. Unfortunately, air and water pollution, detected in many countries with extreme poverty and unfavorable lifestyles, is decreasing very slowly [4, 19–21].
Another worrying aspect related to pollution is the progressive and severe global warming as a direct threat to health. Severe heatwaves and climate changes are associated with increased mortality rates in the elderly and in individuals with chronic cardiorespiratory diseases. These climatic phenomena increase exposure to various risk factors present in the inhaled air, such as pollens, fungi, toxic gases, and particulate matter.
In a parallel event, outdoor pollution increases the amount of pollen grains and chemically modified aeroallergens. Global warming prolongs the vegetation periods of plants and, if followed by extreme climate events like heavy precipitation, provokes a sudden release of massive amounts of allergens. These allergens interact with sensitized mast cells, inducing the release of inflammatory mediators and, thereby, leading to severe asthma attacks [19, 22].
Air pollution is an important component of the exposome [23], which is the set of environmental exposures throughout our lives, with a significant negative impact on our health. The exposome is changing rapidly in recent times due to modifications in the way we work and live [24, 25]
In contrast to this serious situation, the decrease in air contamination, with a reduction of the most harmful components of the exposome, produces substantial, fast, and favorable results in terms of public health. The control of these factors has a positive impact on national, regional, municipal, and even family budgets. This strategic position reinforces the idea that these risk factors are preventable and controllable. US reports revealed that investments of around 65 billion dollars starting in the 1970s, aimed at reducing the impact of pollution, resulted in a budget inflow of 1.5 trillion dollars [5].
External environmental pollution contributes significantly to asthma and other allergic diseases, chronic rhinosinusitis, exacerbations of chronic obstructive pulmonary disease (COPD), respiratory infections, sleep apnea, and several neoplasms, especially lung cancer. On the other hand, indoor smoking and pollution caused by cooking using biomass, kerosene, or diesel derivatives are factors significantly associated with respiratory health aggression. Although tobacco use is decreasing in many countries, a considerable number of children and adolescents start every day with traditional forms of tobacco use or with the more recently introduced variants in Western countries, such as hookah, electronic cigarettes, and heated tobacco devices. Young people rapidly accepted these new devices, which represent a component of pollution that is not yet well known and that must be better studied [14, 19].
Numerous polluting agents have a clear relationship to respiratory disease outcomes, especially asthma and COPD. Among them, PMx, O3, SO2, CO, and NO2 stand out for inducing cough, sputum, and bronchial hyperresponsiveness in many patients. In addition, there is an interference in many outcomes, such as incidence, prevalence, hospital admission, visits to emergency departments, mortality, and asthma attacks, among others.
Due to the importance of the binomial respiratory tract/environmental pollution, this narrative review will focus on polluting agents related to asthma outcomes [2, 13, 26]. However, this paper will not address the relationship between asthma and indoor pollution.
Principal Polluting Agents and their Sources of Emission
Despite the recognition that there is no safe level of pollutants in the air and that exposure even to concentrations below the limits recommended by the WHO may pose a risk, there is still a long way to go towards reducing emissions to an acceptable level [27]. Many countries have established parameters that would be safer for one’s health and agree on the need to update these variables periodically [26]. Measurements over time of the concentrations of some air pollutants, such as carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), particulate matter (PMx), and ozone (O3), serve as markers of the environmental situation in a given area [6].
The human body’s responses to air pollutant aggressions depend on the type, duration, and intensity of exposure, atmospheric conditions, and individual characteristics. Socioeconomic aspects of a specific population may at least partially explain the heterogeneous results detected in a community, city, or even neighborhood [19].
Primary pollutants are those directly released by the emission sources. The most relevant are sulfur dioxide (SO2), nitrogen oxides (NOx), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), black carbon (BC), particulate matter PMx), volatile organic compounds (VOC), and some metals. Secondary pollutant agents are those formed in the atmosphere through chemical reactions between primary pollutants. Examples are hydrogen peroxide (H2O2), sulfuric acid (H2SO4), nitric acid (HNO3), sulfur trioxide (SO3), nitrates (NO3−), sulfates (SO42−), and ozone [13].
The physical-chemical characteristics of these agents are decisive for the type of aggression that will occur in the respiratory system. These pollutants may have additive or synergistic effects with each other, resulting in even more damage than the isolated effects of each component. More studies are needed to better clarify these interactions [2].
The main sources of environmental air pollutants are the results of human action, including automotive vehicles, ships, airplanes, industries, and the burning of biomass [28].
Table 1 shows the different types of pollutants and how they damage human tissues [13].
Table 1.
Different types of air pollutants and the damage in human tissues (adapted from Schraufnagel et al. [13])
| Pollutant | Injury determinants | Tissue affected |
|---|---|---|
| Sulfur dioxide (SO2) | Highly soluble | Upper airway |
|
Nitrogen dioxide (NO2) Ozone (O3) Carbon monoxide (CO) |
Less soluble (NO2 and O3) |
Deeper penetration; bronchial and bronchiolar injury; Tissue hypoxia |
| Particulate matter (PM10, PM2.5, PM0.1) | Size, structure, and composition determine toxicity |
Large particles: mucous membranes and upper airways Small particles: bronchioles and alveoli Ultrafine particles: systemic tissue reactions |
PM0.1 particulate matter with an aerodynamic diameter < 0.1 μm, PM2.5 particulate matter with an aerodynamic diameter < 2.5 μm, PM10 particulate matter with an aerodynamic diameter < 10 μm
Particulate matter (PM10; PM2.5; PM0.1): This group forms one of the main aggressors to health and the environment. The components, suspended in the air, are solid and liquid particles classified by size. PMx is a mixture of chemicals (hydrocarbons, salts, and other compounds given off by vehicles, cooking stoves, and industries) and other natural components, such as dust and microorganisms. PMs may cause damage related to its chemical properties, structure, surface, and composition [13].
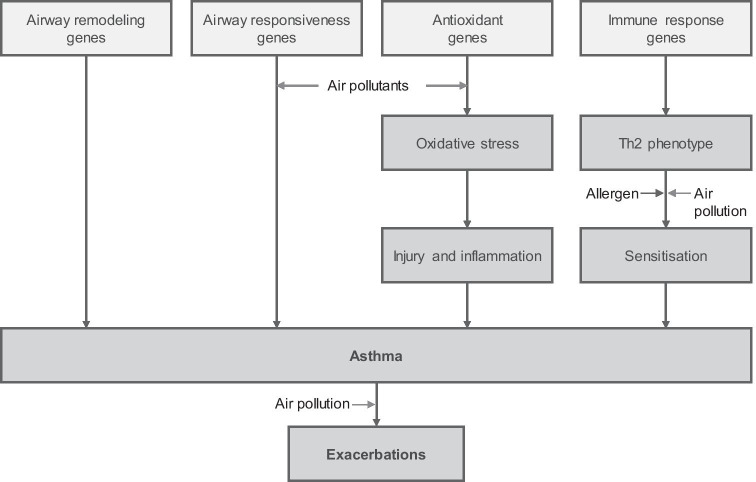
Figure 1 illustrates the main air pollutants according to some physical properties [29].
Fig. 1.
Classification of air pollutants according to some physical properties (modified from Sompornrattanaphan et al. [29])
There are three subgroups: (a) the coarse particles have an aerodynamic diameter ranging from 2.5 to 10 µm (PM10–2.5) and are usually in the upper airways, while those between 4 and 10 µm (MP4–10) are retained in the intermediate regions of the lower airways. The fine particles have an aerodynamic diameter of ≤ 2.5 µm (PM2.5) and reach the bronchiolar and alveolar region. The ultrafine particles/UFP have a diameter smaller than 0.1 µm (PM0.1), and pass over the alveolar barrier to enter the bloodstream, promoting adverse effects in various organs. Ultrafine particulate matter carries harmful adsorbed components to more distal portions of the lungs [30]. When PM monitors are restricted in terms of distribution in a specific area, the obtained data might not be representative of the entire population.
It is worth noting that people with asthma, pneumonia, diabetes, and respiratory and cardiovascular diseases are especially susceptible and vulnerable to the effects of PMx. The particles produce toxic effects according to their chemical and physical properties. The components of PM10 and PM2.5 can be organic (for instance, polycyclic aromatic hydrocarbons, dioxins, benzene, 1-3 butadiene) or inorganic (carbon, chlorides, nitrates, sulfates, metals). PM2.5, followed by PM10, are strongly associated with diverse respiratory system diseases [31], as their size permits them to reach interior spaces [8, 32, 33].
The causal relationship between PM chronic exposure for long years and outcomes of various respiratory diseases has different pathways, depending on each illness, such as asthma, rhinitis, COPD, and lung cancer. Transition metals, polycyclic aromatic hydrocarbons, and environmentally persistent free radicals are constituents of PM of special interest because of their potential to cause oxidative stress and because of the many phenotypic changes associated with asthma. Additionally, particulate matter frequently contains various immunogenic substances, such as fungal spores and pollen, which have been independently associated with the exacerbation of asthma symptoms.
Particulate matter causes the activation of oxidative stress through the production of reactive oxygen species, innate immunity, adaptive immunity, and other mechanisms, leading to the development and exacerbation of respiratory diseases, as will be discussed in a later section of this review [2, 13, 34].
The mechanisms involved suggest the development of persistent oxidative stress and inflammation, which exacerbate chronic diseases or induce their occurrence.
Acute variations in environmental pollutant concentrations are associated with an increase in Emergency Department visits, hospitalizations, and death rates. PM can also interact with allergens in the air and induce asthma exacerbations in previously sensitized people [34].
In summary, substantial evidence supports the idea that ambient levels of PM exacerbate pre-existing asthma, particularly by contributing to oxidative stress and allergic inflammation, and evidence exists in support of PM as a cause of new cases of asthma.
Table 2 shows the consequences of outdoor pollution with respect to allergic rhinitis and asthma [22].
Table 2.
Consequences of outdoor air pollution over allergic rhinitis and asthma (adapted from Eguiluz-Gracia et al. [22])
| Environmental factors | Health outcomes |
|---|---|
| Pollution from traffic and industry (PM10, PM2.5, NO, NO2) | |
| During childhood | Higher asthma prevalence after the school age |
| During adulthood | Possibly higher asthma prevalence |
| Lifelong |
Poorer lung function Higher rate of asthma exacerbations Conflicting results on AR onset |
| Livestock farming (organic dust, toxins form microorganisms, gases like ammonia and methane) | Decreased lung function |
| Black carbon | Possibly epigenetic changes leading to increased type two inflammation in children |
| Interaction between air pollutants (PM10, nitrogen oxides) and allergens (pollen, fungal spores) | |
| Production of more pollen, more allergens per pollen grain, and more PALMs per pollen grain |
Potentially, facilitation of IgE sensitization against aeroallergens Higher rate of asthma-related hospitalizations |
Ultrafine particles UFP/PM0.1: The ultrafine particles (UFP; PM0.1) are airborne particulates of < 0.1 μm in aerodynamic diameter. Typically, UFPs are generated in the environment, often as secondary products of fossil fuel combustion, as the results of condensation of semi-volatile substances, or industrial emissions. Nanoparticles have similar physical characteristics to UFP, but the difference is in the production through industrial bioengineering processes.
Urban air contains large quantities of UFPs, such as components of diesel exhaust particles, products of cooking, and indoor heating using wood incompletely burned in poorly ventilated environments.
Figure 2 is a graphical demonstration of the mechanisms of PM0.1-induced lung diseases proposed by Leikauf et al. [35].
Fig. 2.
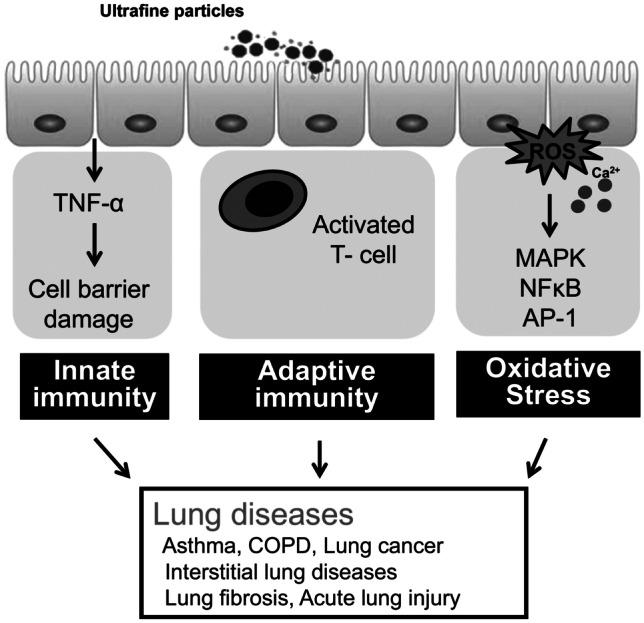
The proposed mechanisms of ultrafine particles induced lung diseases (adapted from Leikauf et al. [35])
Household pollution is associated with several asthma risk factors related to incomplete biomass burning used in domestic cooking without adequate ventilation, as well as to incense sticks, mosquito repellents, and indoor tobacco smoke [36].
The extremely small size of UFPs allows them to bypass host defenses and deposit themselves in the lung, with a high rate of retention. Thus, for the same volume of air inhaled, the actual dose and effects of UFPs in the lung might be significantly greater than that of PM2.5.
There is a relationship between PM0.1 toxicity with its smaller size, larger surface area, and material adsorbed on the surface. These submicron-scale particles have physicochemical properties that are significantly different from those of larger PM and, therefore, might exert adverse health effects, including promoting asthma exacerbation and allergic sensitization to common allergens.
While the health effects of PM10 and PM2.5 are based on their mass, the “weightless’’ nature of UFPs requires a different metric for exposure, such as the particle number and surface area, as shown in Table 3 [30].
Table 3.
Comparison of PM10, PM2.5, and UFP (adapted from Schraufnagel [30])
| Characteristics | PM10 | PM2.5 | UFP |
|---|---|---|---|
| Aerodynamic diameter (mm) | 2.5–10 | 2.5–0.1 | < 0.1 |
| Deposition in alveolar space | No | No | Yes |
| Surface area/mass ratio | + | ++ | +++ |
| Organic carbon content | + | ++ | +++ |
| Elemental carbon content | +++ | ++ | + |
| Metal content | +++ | ++ | + |
| Exposure metrics | Mass | Mass | Particle number or surface area |
| Central monitoring sites | Yes | Yes | None |
| National Ambient Air Quality Standards (NAAQS)/US EPA | 150 mg/m3 (24 h) | 35 mg/m3 (24 h) | None |
Short-term UFP exposure causes various respiratory symptoms, such as coughing and dyspnoea, but also the worsening of spirometric parameters, the greater use of medications to control asthma, and a higher frequency of hospitalizations for asthma. Higher frequency of medical visits for respiratory diseases is also associated with increased levels of PM0.1 [37, 38]. In comparison to PM2.5, UFPs probably cause greater lung inflammation and remain longer in the lungs. Schraufnagel reported that decreased peak expiratory flow and increased respiratory symptoms in asthmatic subjects were associated with exposure to ambient fine and ultrafine particles. However, the effects of the 5-day UFP mean number were larger than that expected by the fine particles, and its effect on peak expiratory flow, FEV1, and forced vital capacity was more intense than that of PM10 [30].
Cereceda-Balic et al. [38] showed that the largest increase in the OR for pediatric asthma-related visits was associated with the 4-day mean concentration of UFPs, while the concentration rises of PM, black carbon, and sulfur reported were lower [39].
However, many findings are still controversial. Weichenthal et al., assessing more than 1 million adults, did not find any association between PM0.1 exposure for long periods, with clinical manifestations, after adjusting for PM2.5 and NO2 [40]. From this same perspective, Clifford et al. stated that the number of ultrafine particles did not show independent association with respiratory symptoms, asthma diagnosis, or changes in lung function [41].
In most of these situations, oxidant injury plays an important role in UFP-induced adverse health effects, including exacerbation and promotion of asthma and chronic obstructive pulmonary disease. The inflammatory properties of PM0.1 and its ability to produce ROS explain these exacerbations, leading to the production of pro-inflammatory cytokines and more airway inflammation.
It is unknown how long UFP persists in human airways and how long a potential pro-allergic effect continues to exist. Schauman et al. observed no significant effects of UFP on allergic inflammation at 24 h after an allergen exposure challenge compared with exposure with filtered air. However, the authors speculate that inhaled UFP particles in real life might have a long-term effect on the inflammatory course in asthmatic patients [42].
The potential for PM0.1 to cause harm to health is great, but their precise role in many illnesses is still unknown.
Gaseous Components (NO2, SO2, O3, CO)
Nitrogen dioxide (NO2): Cooking food with indoor gas stoves releases high NO2 concentrations compared with findings in the outdoor environment.
Immediately after inhalation, NO2 dissolves at the most distal airways and at the alveoli. The next step is the production of reactive oxygen and nitrogen substances (ROS and NOS). These substances induce oxidative stress, damaging the respiratory tract, especially in asthmatics. An increase in respiratory symptoms such as wheezing, dyspnea, and chest tightness might start with very low NO2 exposure [34, 43]. This gas attaches to hemoglobin and other iron-containing proteins, but only if its contact point is in a short distance [13].
Considering that NO2 is one of the major components of TRAP (traffic-related air pollution), such emissions should be mitigated and included in public health asthma control programs. Meta-analyses detected an association between TRAP exposure and pediatric asthma incidence, indicating an association with NO2, but results were mixed for associations with PM2.5. The US Environmental Protection Agency and Health Canada pointed out the causal relationship between long-term NO2 exposure and pediatric asthma [44, 45].
NO2 is a potent irritant of the respiratory system, as it penetrates deep in the lung, inducing respiratory diseases, coughing, wheezing, dyspnea, bronchospasm, and even pulmonary edema when inhaled at high concentrations. It seems that concentrations over 0.2 ppm produce these adverse effects in humans, while concentrations higher than 2.0 ppm affect T lymphocytes, particularly the CD8+ cells and NK cells that produce the human immune response. Long-term exposure to high levels of NO2 can be responsible for chronic lung disease [8].
Sulfur dioxide (SO2): The outdoor release of this gas occurs through the combustion of fossil material, as in automotive vehicles, ships, airplanes, and industrial plants, and through release into the atmosphere by volcanoes. Sulfur dioxide is one of the main sources of acid rain, whose aerosols have pH < 1.0.
The major health problems associated with SO2 emissions in industrialized areas are respiratory symptoms, bronchitis, and mucus production. As it is a sensory irritant and penetrates deep into the lung, this gas interacts with sensory receptors, causing bronchoconstriction.
SO2 is highly soluble in water and largely damages the upper airways and skin. Its effects on the respiratory tract may be short-term, such as respiratory symptoms and increased frequency of emergency room visits and hospitalizations related to respiratory conditions. Long-term exposure causes a decrease in lung function [26, 46–51].
Ozone (O3): O3 is a compound of low water-solubility that reaches the more distal regions of the lungs, achieving the alveolar level. Although ozone in the stratosphere plays a protective role against ultraviolet irradiation, it is harmful in high concentrations at ground level, affecting the respiratory and cardiovascular system [52].
Toxic effects induced by ozone occur in urban areas all over the world, causing biochemical, morphologic, functional, and immunological disorders [53].
Asthmatic individuals probably have a greater susceptibility to the effects of O3 leading to more severe asthma due to an increase in bronchial contractility and an action in the inflammatory cascade [54]. The main consequences of ozone’s inhalation are the increased frequency of asthma exacerbations, the need for more bronchodilator medications, and an increased number of visits to emergency services, even in low external environmental concentrations of ozone. There is a decrease in FEV1 and greater responsiveness during periods of high atmospheric O3 concentrations [43, 55].
Li X et al. recently demonstrated that the better interval for evaluating ozone’s effect on asthma exacerbations is within eight hours of exposure. When assessed using the daily mean concentration, this association does not remain significant [53].
CO: Carbon monoxide is a highly soluble and nonirritating gas that readily passes into the bloodstream. Its toxicity results mainly from its successful competing with oxygen in binding to hemoglobin, which results in tissue hypoxia [13].
Evidence of the acute effects of ambient CO pollution on morbidity risk in developing countries is scarce and inconsistent. Even in the same country, such as China, differing health outcomes, sample sizes, and characteristics of study locations may explain the contradictory result. Liu H et al. detected 916,388 admissions during January 2014 to December 2015. A 1-mg/m3 increase in CO concentrations corresponded to a 4.44% (95% CI, 3.97–4.92%) increase in respiratory admissions on the same day [56]. The associations remained significant after controlling for criteria co-pollutants (PM2.5, PM10, NO2, SO2, and O3). Associations between CO and daily hospital admission for respiratory diseases appeared to be stronger in females and the elderly. The authors speculate that the sex differences in the magnitude of effect may be attributable to hormones and structural/morphological differences in the respiratory system between males and females. Changes in the structure of the respiratory system with advanced age and declining biological function and anti-infection ability may explain the higher effect estimates in the elderly.
The incomplete combustion of fossil fuel produces CO and CO2. Higher amounts of CO2 in the atmosphere increase the duration of pollen seasons, the quantity of pollen produced by plants, and, possibly, the allergenic potential of the pollen. This chain of events has a relationship to stronger IgE binding intensity. The consequence is an increased rate of allergic asthma attacks [57].
Evidence suggests an association between exposure to CO and moderate or severe asthma exacerbations only in adults (OR1.045, 1.005–1.086). Although it was not confirmed in children, during asthma attacks in infants and toddlers, Ohara et al. detected that their exhaled CO levels were significantly higher than those of subjects with asymptomatic asthma (P < 0.0001) and healthy children (P < 0.0001) [57].
Another point to be highlighted is that significant association was observed between decreasing asthma death rates with lower CO levels [46].
Toxic heavy metals: Heavy metals such as lead, arsenic, and cadmium induce local inflammation in the airways and, potentially, systemic effects. In the lungs, they determinate the depletion of antioxidants, and, as a consequence, induce oxidative stress, and an increase in pro-inflammatory agent content [58].
Recently, Koh et al. detected a significant association between heavy metals with asthma, allergic rhinitis, and airflow obstruction, pointing especially at the health consequences of lead, cadmium, and mercury [33].
Epidemiological Findings
Pollution is a real public health problem in both pediatric and adult populations. Outdoor pollution exposure is the fifth leading risk factor for deaths in the world, accounting for 4.2 million deaths, while more than 3.8 million die from situations related to indoor pollution. Considering just the asthma deaths in 2016, the Global Burden of Disease estimated that 420,000 people in the world died from asthma, more than 1000 per day [13]. Worldwide, asthma was the second leading cause of death among chronic respiratory diseases, with a death rate of 6.48/100,000 (4.43–8.39). The Global Asthma Report 2018 informs that the proportion of asthma deaths in all-causes mortality was 0.88% (0.60–1.14) [59].
Table 4 shows the chronic respiratory disease-attributable death rates and DALY rates per 100,000 individuals [60].
Table 4.
Chronic respiratory disease-attributable death rates and DALY rates per 100,000 individuals (adapted from Soriano et al. [60])
| Death rate/100,000 | Proportion all-cause deaths | DALY rate/100,000 | Proportion all-cause DALYS % | |
|---|---|---|---|---|
| All chronic respirat diseases | 51.23 (49.61–52.94) | 7.00% (6.76–7.23) | 1470.03 (1369.68–1566.56) | 4.50% (4.20–4.78) |
| COPD | 41.85 (39.64–43.96) | 5.72% (5.43–5.97) | 1068.02 (994.47–1135.50) | 3.27% (2.96–3.56) |
| Asthma | 6.48 (4.43–8.39) | 0.88% (0.60–1.14) | 297.92 (236.69–370-88) | 0.91% (0.76–1.09) |
| Interstitial lung diseases and pulmonary sarcoidosis | 1.93 (1.50–2.37) | 0.26% (0.20–0.32) | 44.04 (36.19–53.43) | 0.13% (0.11–0.16) |
| Pneumoconiosis | 0.28 (0.27–0.30) | 0.04% (0.04–0.04) | 6,64 (6.18–7.17) | 0.02% (0.02–0.02) |
| Other chronic respiratory diseases | 0.68 (0.60–0.78) | 0.09% (0.08–0.11) | 53.40 (47.16–59.63) | 0.16% (0.05–0.18) |
However, as compared with other chronic respiratory diseases, asthma mortality is not frequent. It is usually attributed to several risk factors, but the majority are preventable and related to the bad management of asthma [61, 62].
The asthma mortality rate, evaluated in 46 countries, decreased from 0.44 deaths/100,000 (0.39–0.48) in 1993 to 0.19/100,000 (0.18–0.21) in 2006, though without a significant difference in the following period, 2006–2012 [61].
Air pollution plays a role in asthma mortality. Liu Y et al. included 4454 cases of asthma deaths during the study period (2013–2018), with an average of at least two asthma death cases per day. The authors found an 11% increase in asthma deaths related to an increase of NO2 concentration over the 3 days before death occurred. They also reported that following increases in exposures to PM2.5 and O3, the OR for asthma mortality increased by 7% and 9%, respectively. This report concludes that an association exists between asthma deaths and short-term air pollution exposure [31].
In a meta-analysis, Veremchuk et al., studying an urban population, reported that an association between asthma morbidity and air pollution was stronger in children than in adolescents and adults [63].
The role that environmental pollution plays in the various asthma outcomes is becoming clearer, not only in mortality but also in hospitalizations, Emergency Department visits, exacerbations, and others, with the advance of the understanding of the deleterious effects of the polluting agents. The Asthma Global Burden Report estimated that asthma-related emergency room visits occurred between 9–23 million and 5–10 million of attributable to ozone and PM2.5, respectively. Those rates represented 8–20% and 4–9% of the annual number of global visits, respectively [64].
The prevalence of asthma is increasing worldwide, especially in densely industrialized urban regions. Cross-sectional and longitudinal studies show the association between asthma and exposure to air pollutants [46, 65]. Previously, in 2006, Watts had already described physicians’ concern about the great increase in asthma incidence in China after the industrial development of the last decades, likely associated with a significant increase in the concentration of pollutants [66].
Numerous polluting agents exacerbate respiratory diseases, especially asthma and COPD. Among them, PMs, O3, SO2, CO, and NO2 drew attention and were associated with respiratory symptoms such as cough, sputum, and bronchial hyper-responsiveness. Even short-term exposures to high concentrations of environmental pollutants are associated with reduced lung function, exacerbations of asthma, and higher frequencies of visits to emergency departments, hospitalizations, and deaths [64].
Pediatric Asthma
It is generally difficult to quantify the effects of an individual pollutant and respiratory diseases without multi-pollutant models. This difficulty might explain some inconsistent findings between ambient pollutants and respiratory diseases.
Dong et al. published a study in 2011 evaluating 30,139 Chinese children aged 3 to 12 years, stratified by their allergic predisposition. The results showed that allergic children were more susceptible to air pollutants than the control group, and that this was more frequent among females. They also detected a strong correlation between PM10 and SO2 (r = 0.78), PM10 and NO2 (r = 0.70), PM10 and O3 (r = 0.74), SO2 and O3 (r = 0.67), and NO2 and O3 (r = 0.66). These findings prove that it is difficult to distinguish the effects of individual air pollutants [67].
Achakulwisut et al. [68] studied the annual burden of pediatric asthma incidence attributable to ambient NO2 pollution, evaluating the intra-urban and near-roadway exposure in 125 major cities of 194 countries. Their findings suggested that a substantial portion of pediatric asthma incidence could be avoided by reducing NO2 pollution in both developed and developing countries, especially in urban areas. Anenberg et al. also mentioned the importance of NO2 in asthma as a public health problem [64].
In a recent large study, Lee et al. detailed 28,824 asthma exacerbations that required hospital admission and the lag period after exposure. They evaluated air pollutants, weather, aeroallergens, respiratory viral infections, and the effect in five age groups (infants, preschool children, school-aged children, adults, and the elderly). The conclusion was that asthma exacerbations were associated with O3, daily temperature range, and tree pollen on lag day 0; with PM10, NO2, CO, and influenza virus infection on lag day 3; and with SO2 and weed pollen on lag day 5 [69].
Zhao Y et al. studied during the period January 2013 to August 2017 the data records of 89,484 hospital outpatient visits for respiratory diseases. They found that a short-term exposure to ambient CO was associated with an increased risk of outpatient visits for respiratory diseases. In asthma, an increase in ambient CO corresponded to an increased risk in outpatient visits for asthma of 8.86% (95% CI 4.89%, 12.98%) [70].
A study conducted with children in Athens, Greece, aimed to assess the effects of acute exposure to air pollutants, revealed that an increase of 10 μg/m3 in MP10 and SO2 levels was associated with the increments of 2.2% (95% CI 0.1–5, 1) and 6.0% (95% CI 0.9–11.3), respectively, for asthma in emergency services [71].
Another publication on children and adolescents up to 18 years old described a higher frequency of hospitalizations for asthma associated with increases in NOx concentrations (OR 1.11; 95% CI: 1.05–1.17), NO2 (OR1.10; 1.04–1.16), MP10 (OR 1.07; 1.03–1.12), and MP2.5 (OR 1.09; 1.04–1.13) [72].
In Araraquara, Brazil, a city located in the sugarcane crop region, hospitalizations for asthma increased with the augmentation of pollutant levels. During the straw burning period, hospital admissions for asthma, at all ages, were around 50% higher. An increment of 10 μg/m3 in the concentration of total suspended particles was associated with an increase of 11.6% (5.4–17.7) in hospital admissions for asthma [51]. Another study carried out in Brazil found that after a period of forest burning, when the concentrations of MP2.5 reached values of up to 400 µg/m3, there was a 100% increase in emergency care for children under 10 years of age due to respiratory diseases, including asthma [73].
Pollution seems to be related to worse outcomes not only for patients with a previously confirmed asthma diagnosis but also for the emergence of new cases, that is, an increase in the incidence of the disease. Growing evidence confirms that inhalation of pollutants in childhood is associated with an increased risk of developing asthma and impairment of the development of normal lung function [52, 74–76].
Several reports show increases in the frequency of new cases of asthma in childhood associated with continued exposure to pollutants generated by the burning of fossil fuels, such as black carbon, PM2.5, and NO2 [54, 55, 77].
In a comprehensive assessment of the respiratory consequences of forest fires in San Diego, USA, in 2007, there were 21,353 hospitalizations, 25,922 emergency visits, and 297,698 outpatient consultations. There was a 34% increase in all respiratory diagnoses, but asthma increased by 112%. Among children under 4 years old and those up to 1 year old, Emergency Department visits for asthma increased 70% and 243%, respectively. An increase in the concentration of 10 μg/m3 of PM2.5 had, as a consequence, OR of 1.08 (95% CI 1.04–1.13) for emergency care for asthma [78].
The Southern California Children Health Study (CHS), conducted in 12 communities in California, USA, with different levels of ozone concentration, included 3535 students with no previous history of asthma. The researchers followed these volunteers for 5 years and found that 265 children developed asthma. In communities with higher ozone concentrations, the OR for asthma development among children who practiced outdoor sports three or more days per week was 3.3 (95% CI 1.9–5.8) times greater than that of children who did not play sports. In areas with low O3 concentrations, outdoor sport practice was not a significant risk factor for asthma appearance. The same type of result was previously observed considering the length of stay in external environments; only permanence in areas with a high concentration of O3 was directly associated with new cases of asthma [52].
In the Netherlands, a study following 3863 children for 8 years reported an increase in the prevalence and incidence of asthma of 28% and 26%, respectively; this was associated with exposure of vehicular origin, especially with increasing concentrations of MP2.5 [79].
To assess a possible relationship between asthma incidence rates with pollution, a study evaluated the emergence of new cases in people up to 18 years of age in 48 US states and the District of Columbia, in two waves (2000 and 2010). The authors estimated that the number of asthma cases attributed to vehicular pollution ranged from 209,100 to 331,200 in 2000, falling to 141,900 to 286,500 in 2010. The possible cause of such a drop was the significant reduction in NO2 and PM concentrations, evidencing not only the association of exposure to the disease but also the benefits of reducing pollutants [80].
Another study, with similar objectives, reported data from 194 countries. The authors estimated that in 2015 four million new cases of asthma in individuals aged 18 years or less were related to exposure to NO2. That number represented about 13% of the global annual asthma incidence [68].
There was previous evidence that the MP10 fraction could be more harmful to the airway epithelium than PM2.5, possibly related to the iron content of these larger particles. In animal models, Herbert et al. [81] demonstrated a clear cause-and-effect relationship between rates of PM10 in ambient air and allergic asthma, for both asthma induction and exacerbation. However, another study detected a different result, that is, greater evidence of the effect of PM2.5 concentrations [82]. Probably, the variation in particle composition between studies is the main reason for such different findings.
The effect of urbanization on the increase of the frequency of new asthma cases, demonstrated in cross-sectional studies and longitudinal studies, strengthened the hypothesis of the direct interference in the immune system and, consequently, in the prevalence of asthma [83]. After one moves to an urban area, daily habits usually change, with families spending more time indoors, especially at home or offices. The World Health Organization reports that in urban settings, individuals can spend up to 90% of their time in household environments. In addition, data show that such domestic environments are two to five times more polluted than outdoor areas [1].
Cross-sectional studies show that when inhabitants of rural areas acquire urban lifestyles or move to more densely populated centers, there is an increased frequency of wheezing patients, probably related to their breathing more polluted air. In addition, several longitudinal studies specifically analyzed the phenomenon of urbanization through air pollution related to urban traffic and found an association with increased incidence, prevalence, and exacerbation of asthma in children and adults [83–88].
In 2013, the study Improving Knowledge and Communication for Decision Making on Air Pollution and Health in Europe (APHEKOM), assessing the impact of children living close to high-traffic routes in 10 European cities, revealed that air pollution was responsible for up to 14% of all asthma patients and for 15% of asthma exacerbations [86].
It is now clearer that TRAP plays a special role in air pollution and health aggression. The smoke from burning diesel oil (diesel exhaust particles) contributes up to 90% of the PMs that constitute TRAP. These components induce oxidative stress and bronchial hyperresponsiveness, as well as increase allergic responses and inflammation in the airways. In addition, TRAP is associated with reduced lung growth and asthma [75, 75].
The ESCAPE study evaluated five cohorts of newborns in several countries in Europe, aiming to study the association between TRAP exposure and childhood asthma or wheezing. The authors initially did not detect a significant association between PM and NOx with the prevalence of asthma or current wheezing in childhood [75]. However, a data reanalysis, which resulted in several publications, found that exposure to those pollutants was associated with worse lung function in children aged 14 to 16 years. In addition, also described was the relationship between exposure to PM2.5 and NO2 with a higher incidence of asthma. The OR for asthma incidence were 1.29 (95% CI 1.00–1.66) and 1.13 (95% CI 1.02–1.25), respectively [75, 89, 90].
Evidence suggests that 13% of the global incidence of asthma in children could be attributable to TRAP and data showed that air pollution has a negative impact on asthma outcomes in both adult and pediatric populations. The Global Initiative for Asthma (GINA) includes this issue in the 2020 update [59, 91].
Khreis et al., in a systematic review and meta-analysis of 41 studies, showed a significant effect of exposure to black carbon (BC), NO2, PM2.5, and PM10 on the risk of developing asthma in children under 18 [84]. After that publication, several papers demonstrated that a high concentration of NO2 and BC in urban areas showed the important relationship of heavy traffic with higher prevalence and incidence of asthma in children [80, 88, 92–94].
Exposure to NO2 and NOx derived from urban traffic is responsible, yearly, to 7% and 12% of new asthma cases, respectively. Exposure to these pollutants from industries, heating equipment, aviation, and several others added to TRAP composition increase the proportion to 22% and 35%, respectively [93].
The same group of researchers confirmed that the TRAP-related pollutants PM2.5, PM10, and BC play a partial role in 7%, 11%, and 12% of the annual incidences of childhood asthma, respectively [80]. The percentages related to these same pollutants, considering all other sources, would be 27%, 33%, and 15%, respectively [76].
The longitudinal assessment of the incidence of asthma in childhood allows for the evaluating of the number of preventable cases if the levels of the pollutants were in accordance with WHO recommendations, as suggested by a nationwide study carried out in the USA (80). In the same year, Khreis et al. [76] assessed the association between air pollution and the emergence of new cases of asthma in more than 63 million children in 18 European countries. They reported the possible prevention of emergence per year of 2,434 and 66,567 new cases of asthma if the levels of NO2 and PM2.5, respectively, in those countries were within the standards set for air quality by WHO [1, 26, 27, 95]. If those European countries obeyed the suggested limits for black carbon, in addition to NO2 and PM2.5, the annual decrease would be 135,257 (23%), 191,883 (33%), and 89,191 (15%) cases, respectively. The authors pointed out that the WHO parameters should be outdated and suggest that such a correction probably would describe a more realistic asthma burden incidence.
Table 5 shows the effects of outdoor air pollutants on asthma if legal concentrations are exceeded [96].
Table 5.
Effects of outdoor air pollutants on asthma outcomes if legal concentrations are exceeded (adapted from Tiotiu et al. [96])
| Pollutant | Concentration (µg/m3) | Asthma symptoms | Exacerbations | Hospitalizations | Lung function |
|---|---|---|---|---|---|
| O3 | 100 (8-h mean) | - | ↑ | ↑ | ↓ |
| NO2 | 200 (1-h mean) | ↑ | ↑ | ↑ | ↓ |
| CO | 30 (1-h mean) | - | ↑ | - | |
| SO2 | 20 (24-h mean) | ↑ | ↑ | ↑ | ↓ |
| PM2.5 |
10 (annual mean) 25 (24-h mean) |
↑ | ↑ | ↑ | ↓ |
| PM10 |
10 (annual mean) 50 (24-h mean) |
↑ | ↑ | ↑ | ↓ |
Confounding factors, such as socioeconomic status, smoking, and family atopy, may interfere with the assessment of the emergence of new cases of childhood asthma. Unfortunately, many studies did not control those variables.
Adult Asthma
Studies about the associations between outdoor air pollution and asthma in adults are not as common as they are in children, and the underlying biological mechanisms are not completely understood [47, 97, 98].
When the exposure is later, in adulthood, the conclusions are less definitive, although some studies suggested that an association of new cases of asthma with such exposure might become a risk factor for accelerated decline in adult spirometric values [99–101].
Kunzli et al., in one of the first studies looking for an association between adult asthma and air pollution, evaluated 2725 nonsmoking individuals aged between 18 and 60 years old. The results showed that residents in more polluted areas had a higher risk of developing asthma, around 30% for each increase of 1 μg/m3 in the MP10 concentration [92].
More recently, Koh Y et al. [33] reported the results of a study with adults (> 19 year old) assessing the Korean database population looking for data on heavy metal serum levels. The authors assessed 16,809 adults with asthma and atopic dermatitis, 9547 with allergic rhinitis and allergic multimorbidities, and 8092 with complete pulmonary function testing. Their results indicated that serum lead level was associated with self-reported asthma (aOR 1.10; 1.02–1.17) and atopic dermatitis (aOR 1.12; 1.02–1.23), cadmium level was associated with self-reported asthma (aOR1.36; 1.19–1.55) and allergic rhinitis (aOR 1.11; 1.03–1.19), and mercury level was not associated with any of the studied allergic conditions.
In a study with 650,000 participants from three European cohorts, Cai et al. [94] reported the relationship between pollution and the prevalence of asthma in adults. They found that prolonged exposure to PM10 was associated with an increase of 12.8% in the prevalence of asthma.
Orellano et al., in a meta-analysis design, considered the most important outdoor air pollutants to be PM, O3, SO2, NO2, CO, and Lead (Pb), with a significant association between several of those pollutants and gases with moderate or severe exacerbations of asthma [46].
Bowatte G et al. investigated the associations between nitrogen dioxide (NO2) exposure, traffic road, and persistent asthma, following the patients over eight years. Living close to a major road was a risk factor for the development and persistence of asthma in adults. For those who never had asthma by age 45, living < 200 m from a major road presented increased odds of new and persistent asthma (aOR 5.20; 95% CI: 1.07, 25.4). Asthmatic participants at 45 also had an increased risk of persistent asthma up to 53 years if they lived < 200 m from a major road, compared with asthmatic participants living > 200 m from a major road (aOR 5.21; 95% CI 1.54, 17.6) [102].
Havet et al. found increases in adult plasma levels of fluorescent oxidation products (FLOPs) after O3 and PM10 exposures and that this increase was associated with a risk for persistent asthma. These findings strengthen the role that FLOP levels play in inducing oxidative stress [103]. These findings are in accordance with previous studies of uncontrolled asthma [98], current asthma [97], and severe asthma in adults [104].
Recent papers addressed various aspects of asthma in adults and exposure to polluting agents. Liu Y et al. found that short-term exposures to PM2.5, NO2, and O3 probably increase the risk of adult asthma mortality [31]. Scibor et al. reported, in 2020, an association between exposures to PM10 and worse quality of life in adult asthma patients [105].
Improvement in Air Quality and the Impact on Asthma Cases
Several studies noted the relationship between the reduction of external environmental pollution and the improvement of respiratory conditions. Evidence of improvements in air quality concomitant with a decrease in asthma cases occurs in the short term and long term.
Garcia et al. [106] reported that the improvement in air quality in Southern California between 1993 and 2014 was associated with a lower incidence of childhood asthma, a conclusion that remained even after controlling for several possible confounding factors. The study included 4,140 children, with no previous history of asthma. The researchers identified 525 new cases of asthma and found, in the respective communities of each case, a progressive decrease in the levels of pollution measured in three assessments throughout the study. The relative risk for reducing the incidence of asthma was 0.83 cases/100 persons/year for each decrease of 4.3 ppb of NO2, and 1.53 cases/100 persons/year for each decrease of 8.1 μg/m3 of PM2.5. These results pointed to a reduction in the incidence of asthma by about 20% in the region during the studied period. The results of this and other studies indicate the possible benefits of reducing the concentrations of polluting agents to values below the maximum limits adopted.
A very impressive result was obtained through a major effort during the 1996 Olympic Games in Atlanta, USA, to reduce urban pollution. During the period of the Games, traffic was prevented from circulating in various areas of the city, with a measured decrease of around 22%. This administrative action was followed by a drop in the daily peak of the levels of O3 (− 28%), NO2 (− 7%), CO (− 19%), and MP10 (− 16%) compared with the previous 3 weeks and after the games. In this period, there was a 40% reduction in medical visits among asthmatic children and an 11–19% decline in asthma care at all ages in emergency services [107].
Likewise, during the 2008 Beijing Olympic Games, there was a drop in concentrations of MP2.5 and O3 from 78.8 μg/m3 to 46.7 μg/m3 and from 65.8 to 61 ppb, respectively, as well as a 41.6% decrease in asthma care in emergency services [108].
NASA pollution-monitoring satellites and the European Space Agency detected significant decreases in NO2 levels across China compared with before and during the pandemic quarantine. The reduction of NO2 pollution was visible first near Wuhan; eventually, NO2 dropped across China and around the world. The concentrations of nitrogen dioxide were 10–30% lower than in comparable periods in 2019. The European Environment Agency found a similarly large drop in air pollution across European cities, such as a NO2 decrease of 47% in Bergamo, Italy and 55% in Barcelona, Spain, as compared with the same period in 2019.
Berman and Ebisu evaluated the impact of the COVID-19 pandemic on measured USA air pollution using the federal air-monitoring network. They detected a sharp decrease in NO2 levels (25.5% reduction with absolute reductions 4.8 ppb). The PM2.5 reduction was significant (0.7 μg/m3 or 11.3%) when examining counties that instituted early non-essential business closures, while rural counties did not indicate any statistically significant difference between current and historical data [109].
A decrease of 6% in global pollution followed the reduction of global activities due to the coronavirus pandemic. However, the impact of such a reduction in asthma and COPD patients is just beginning to be evaluated [110].
Main Pathophysiological Pathways
Different responses to exposure to the same polluting agents are related to several intrinsic factors of each subject and extrinsic factors. The intrinsic factors most frequently studied are age, sex, pre-existing diseases, diet, obesity, and viral infections. The genetic load has a crucial role. The polymorphisms recognized in the control of oxidative stress are NQO1, GSTM1, and GSTP1, while the TNF has a role in inflammatory mechanisms.
However, extrinsic factors, such as climate and environment changes, individual socioeconomic restrictions, income differences among countries, cities, or areas, and nutritional status, play a significant role [22, 69, 111].
Ozone, NO2, and PM2.5 are the pollutant agents related to airway inflammation, while ozone and nitrogen dioxide produce airway hyperresponsiveness. In addition, oxidative stress has been associated with ozone, NO2, and PM2 [5]. Through several pathways, these pollutants are associated with exacerbations, mortality, and even the onset of asthma.
Gowers et al. [77] described several mechanisms to explain the role of outdoor pollution in the induction of asthma. Among them, the authors identified airway damage and remodeling through oxidative stress plus inflammatory pathways and immunological responses. In addition, they also considered the enhancement of respiratory sensitization to aeroallergens decreasing the airway hyperreactivity threshold. There is an intense interconnection among these mechanisms [28], as schematically shown in Fig. 3.
Fig. 3.
The interrelation between the various components of the binomial air pollutants in asthma (Guarnieri et al. [28])
A basal mechanism leading to the development of several lung diseases associated with air pollutants is the structural rearrangement of the airway epithelium, extracellular matrix, smooth muscle, and cells involved with immune responses leading to the inflammatory process. The local inflammation occurs with infiltrations of neutrophils and macrophages. These cells start to secrete pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNFα) and interleukins 6 (IL6) and 8 (IL8). TNFα and interferon-gamma (INFγ) increase nitric oxide synthesis, which is another source of free oxygen radicals.
Free radicals and lung inflammation represent a response against the pollutant agents. However, when there is a large production of reactive oxygen and nitrogen species (ROS and NOS), it becomes an adverse effect [112]. Recent studies suggest the environmentally persistent free radicals (EPFR) as another important component that can last in the ambient up to 21 days [113].
This chain of events results in an inflammatory state responsible for exacerbations or worsening in asthmatic patients when exposed to pollutant agents [114]. Animal studies, such as the exposure of rats to ozone, in environments without allergens, induce type 2 immunological reactions, as a non-allergic asthma response. These responses are not the classic type 1 reactions, usually involved in allergic rhinitis and asthma, and amplify the understanding of the relationship between pollution and asthma [88].
Patients with severe asthma produce greater amounts of cytokines when exposed to PMs and diesel exhaustion as compared with healthy individuals or non-severe asthma phenotypes [22].
The reduced function of Treg lymphocytes and increased IgE levels is another point in the complex relationship between air pollution and atopic diseases. A different mechanism plays a central role in non-atopic asthma, which is an increment of CD4 and CD8 T lymphocyte production in response to antigens in polluted environments [28, 115].
Infections by adenovirus and other pathogens can also interact with oxidative stress and pollutant particles, thus inducing disease exacerbation in patients with chronic respiratory disease. This is especially important when considering the role that ultrafine particles play in asthma. UFP is known to act as a carrier of microorganisms to deep regions of the lungs [116].
In addition, air pollution interacts with plants and fungi, promoting an increase in the production of pollens and their respective allergenic capacity. For example, in areas with a high concentration of CO2, ragweed grows faster, flourishes earlier, and, thus, produces more pollen than ragweed in clean-air rural areas. A prick test performed with extracts obtained from pollens in polluted areas produces dermal reactions greater than those from unpolluted areas [111]. Cakmak et al. [117] reported the relationship between air pollution and the risk of hospitalization for asthma with pollens and fungal spores endorsing this situation. Guilbert et al. [118] confirmed the association of exposure to PM10 and aeroallergens with hospitalizations for asthma.
Human airway epithelium seems to play an important role in the initiation and control of the innate immune responses to different types of environmental factors contributors to asthma pathogenesis, such as allergens, microbes, or pollutants [119].
It is accepted that epigenetic mechanisms play a pivotal role in the regulation of different cell populations leading T and B cells to participate in the pathogenesis of asthma. The effects of environmental factors on the development of asthma are mediated, at least in part, by DNA methylation and histone modifications [119].
The common feature of such mechanisms is that they induce asthma without affecting the nucleotide sequence of the genomic DNA, in accordance with the classic definition of epigenetic mechanisms [120].
Thus, through these various routes, exposure to pollutants increases asthma outcomes in all recognized asthma subtypes.
In summary, air pollutants might cause oxidative injury to the airways that leads to inflammation and remodeling, which, in a genetically predisposed individual, could result in clinical asthma. One predisposing factor might be atopy, and air pollutants could increase the risk of sensitization and the responses to inhaled allergens in individuals with asthma.
Conclusions
Exposure to pollutants in inspired air is one of the many clinical points to investigate during a medical interview for the diagnosis of asthma. It must be considered in each individual case, even knowing that there is rarely only a single cause. This interference or causality can occur in allergic or non-allergic asthma, confirming the concept of asthma as a multifactorial disease.
Air pollution plays a significant role in asthma morbidity and mortality rates. The impact is increasing while many countries have not taken effective actions to reduce emissions of these toxic agents.
One of the relevant impacts of air pollution is the association with the increased incidence, prevalence, and exacerbation of asthma, as shown in this narrative review. Exposure to industrial pollution and traffic-related pollutants is an important risk factor for the main outcomes in childhood asthma. However, in adults, the conclusions are less definitive, but additional studies are gradually clarifying our understanding in this point.
The mechanisms through which pollutants induce asthma and other respiratory diseases are multiple, but the key seems to be the inflammatory cascade. There is evidence that epigenetic changes occurring in the respiratory tract microbiome play a role in the pathophysiology of these clinical conditions.
The advance in science on the harmful effects of pollution on respiratory diseases, especially asthma, has not yet experienced the necessary diffusion, even among professionals in the various health professions. However, certainly, new and important advances will lead us to disseminate information about the importance of environmental pollution for all humankind.
Funding
The authors claim that they have not received any form of funding related to this article.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Research involving Human Participants and/or Animals
This paper did not involve human participants or animals.
Informed Consent
As this review did not include any human or animal participants, there was no need of informed consent.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2018) Ambient (outdoor) air pollution 2018. https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health. Accessed 25 Feb 2020
- 2.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, Thurston GD, To T, Vanker A, Wuebbles DJ. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: air pollution and organ systems. Chest. 2019;155(2):417–426. doi: 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, 3rd, Apte JS, Brauer M, Cohen A, Weichenthal S, Coggins J, Di Q, Brunekreef B, Frostad J, Lim SS, Kan H, Walker KD, Thurston GD, Hayes RB, Lim CC, Turner MC, Jerrett M, Krewski D, Gapstur SM, Diver WR, Ostro B, Goldberg D, Crouse DL, Martin RV, Peters P, Pinault L, Tjepkema M, Donkelaar A, Villeneuve PJ, Miller AB, Yin P, Zhou M, Wang L, Janssen NAH, Marra M, Atkinson RW, Tsang H, Thach TQ, Cannon JB, Allen RT, Hart JE, Laden F, Cesaroni G, Forastiere F, Weinmayr G, Jaensch A, Nagel G, Concin H, Spadaro JV. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A. 2018;115(38):9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA, 3rd, Shin H, Straif K, Shaddick G, Thomas M, Dingenen R, Donkelaar A, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Baldé AB, Bertollini R, Bose-O'Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potočnik J, Preker AS, Ramesh J, Rockström J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, Schayck OCP, Yadama GN, Yumkella K, Zhong M. The Lancet Commission on pollution and health. Lancet. 2018;391(10119):462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- 6.Cromar KR, Duncan BN, Bartonova A, Benedict K, Brauer M, Habre R, Hagler GSW, Haynes JA, Khan S, Kilaru V, Liu Y, Pawson S, Peden DB, Quint JK, Rice MB, Sasser EN, Seto E, Stone SL, Thurston GD, Volckens J (2019) Air pollution monitoring for health research and patient care. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc 16(10):1207–1214. 10.1513/AnnalsATS.201906-477ST [DOI] [PMC free article] [PubMed]
- 7.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, Coelho MSZS, Saldiva PHN, Lavigne E, Matus P, Ortega NV, Garcia SO, Pascal M, Stafoggia M, Scortichini M, Hashizume M, Honda Y, Hurtado-Díaz M, Cruz J, Nunes B, Teixeira JP, Kim H, Tobias A, Íñiguez C, Forsberg B, Åström C, Ragettli MS, Guo Y-L, Chen B-Y, Bell ML, Wright CY, Scovronick N, Garland RM, Milojevic A, Kyselý J, Urban A, Orru H, Indermitte E, Jaakkola JJK, Ryti NRI, Katsouyanni K, Analitis A, Zanobetti A, Schwartz J, Chen J, Wu T, Cohen A, Gasparrini A, Kan H. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381(8):705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Environment Agengy (2018) Air quality in Europe: 2018 report. https://www.eea.europa.eu/publications/air-quality-in-europe-2018. Accessed 25 Feb 2020
- 10.Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anenberg SC, Bindl M, Brauer M, Castillo JJ, Cavalieri S, Duncan BN, Fiore AM, Fuller R, Goldberg DL, Henze DK, Hess J, Holloway T, James P, Jin X, Kheirbek I, Kinney PL, Liu Y, Mohegh A, Patz J, Jimenez MP, Roy A, Tong D, Walker K, Watts N, West JJ (2020) Using satellites to track indicators of global air pollution and climate change impacts: lessons learned from a NASA-Supported Science-Stakeholder Collaborative. Geohealth. 4(7):e2020GH000270. 10.1029/2020GH000270 [DOI] [PMC free article] [PubMed]
- 12.Sorek-Hamer M, Just AC, Kloog I. Satellite remote sensing in epidemiological studies. Curr Opin Pediatr. 2016;28(2):228–234. doi: 10.1097/MOP.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, Thurston GD, To T, Vanker A, Wuebbles DJ. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies' Environmental Committee, Part 1: the damaging effects of air pollution. Chest. 2019;155(2):409–416. doi: 10.1016/j.chest.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurston GD, Kipen H, Annesi-Maesano I, Balmes J, Brook RD, Cromar K, Matteis S, Forastiere F, Forsberg B, Frampton MW, Grigg J, Heederik D, Kelly FJ, Kuenzli N, Laumbach R, Peters A, Rajagopalan ST, Rich D, Ritz B, Samet JM, Sandstrom T, Sigsgaard T, Sunyer J, Brunekreef B. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework Eur Respir J. 2017;49(1):1600419. doi: 10.1183/13993003.00419-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponte EV, Cruz AA, Athanazio R, Carvalho-Pinto R, Fernandes FLA, Barreto ML, Stelmach R. Urbanization is associated with increased asthma morbidity and mortality in Brazil. Clin Respir J. 2018;12(2):410–417. doi: 10.1111/crj.12530. [DOI] [PubMed] [Google Scholar]
- 16.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proc Am Thorac Soc. 2010;7(2):102–106. doi: 10.1513/pats.200908-083RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulin M, Simoni M, Viegi G, Annesi-Maesano I. Respiratory health and indoor air pollutants based on quantitative exposure assessments. Eur Respir J. 2012;40(4):1033–1045. doi: 10.1183/09031936.00159011. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (2018) Household air pollution and health. https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health. Accessed 25 Feb 2020
- 19.Haahtela T, von Hertzen L, Anto JM, Bai C, Baigenzhin A, Bateman ED, Behera D, Bennoor K, Camargos P, Chavannes N, Sousa JC, Cruz A, Teixeira MC, Erhola M, Furman E, Gemicioğlu B, Diaz SG, Hellings PW, Jousilahti P, Khaltaev N, Kolek V, Kuna P, Grutta S, Lan LTT, Maglakelidze T, Masjedi MR, Mihaltan F, Mohammad Y, Nunes E, Nyberg A, Quel J, Rosado-Pinto J, Sagara H, Samolinski B, Schraufnagel D, Sooronbaev T, Eldin MT, To T, Valiulis A, Varghese C, Vasankari T, Viegi G, Winders T, Yañez A, Yorgancioğlu A, Yusuf O, Bousquet J, Billo NE. Helsinki by nature: the nature step to respiratory health. Clin Transl Allergy. 2019;9:57. doi: 10.1186/s13601-019-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez A, Brickley E, Rodrigues L, Normansell RA, Barreto M, Cooper PJ. Urbanisation and asthma in low-income and middle-income countries: a systematic review of the urban-rural differences in asthma prevalence. Thorax. 2019;74(11):1020–1030. doi: 10.1136/thoraxjnl-2018-211793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribeiro-Silva RC, Malta DC, Rodrigues LC, Ramos DO, Fiaccone RL, Machado DB, Barreto ML. Social, environmental and behavioral determinants of asthma symptoms in Brazilian middle school students; a national school health survey (Pense 2012) Int J Environ Res Public Health. 2018;15(12):2904. doi: 10.3390/ijerph15122904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eguiluz-Gracia I, Mathioudakis AG, Bartel S, Vijverberg SJ, Fuertes E, Comberiati P, Cai YS, Tomazic PV, Diamant Z, Vestbo J, Galan C, Hoffmann B. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. 2020;75(9):2170–2184. doi: 10.1111/all.14177. [DOI] [PubMed] [Google Scholar]
- 23.Agache I, Miller R, Gern JE, Hellings PW, Jutel M, Muraro A, Phipatanakul W, Quirce S, Peden D. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a Practall document. Allergy. 2019;74(3):449–463. doi: 10.1111/all.13690. [DOI] [PubMed] [Google Scholar]
- 24.Cecchi L, D'Amato G, Annesi-Maesano I. External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol. 2018;141(3):846–857. doi: 10.1016/j.jaci.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Abramson MJ, Guo Y. Indoor endotoxin exposure and ambient air pollutants interact on asthma outcomes. Am J Respir Crit Care Med. 2019;200(6):652–654. doi: 10.1164/rccm.201904-0842ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler A (2018) U.S. Environmental Protection Agency: year in review 2018. https://www.epa.gov/sites/production/files/2019-01/documents/epa_2018_yearinreview_0128-4.pdf. Accessed 22 Jan 2020
- 27.World Health Organization (2006) Air quality guidelines: global update 2005. http://www.euro.who.int/__data/assets/pdf_file/0005/78638/E90038.pdf?ua=1. Accessed 23 Jan 2020
- 28.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sompornrattanaphan M, Thongngarm T, Ratanawatkul P, Wongsa C, Swigris JJ (2020) The contribution of particulate matter to respiratory allergy. Asian Pac J Allergy Immunol 38(1):19–28. 10.12932/AP-100619-0579 [DOI] [PubMed]
- 30.Schraufnagel DE. The health effects of ultrafine particles. Exp Mol Med. 2020;52(3):311–317. doi: 10.1038/s12276-020-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Pan J, Zhang H, Shi C, Li G, Peng Z, Ma J, Zhou Y, Zhang L. Short-term exposure to ambient air pollution and asthma mortality. Am J Respir Crit Care Med. 2019;200(1):24–32. doi: 10.1164/rccm.201810-1823OC. [DOI] [PubMed] [Google Scholar]
- 32.Beamer PI. Air pollution contributes to asthma deaths. Am J Respir Crit Care Med. 2019;200(1):1–2. doi: 10.1164/rccm.201903-0579ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh HY, Kim TH, Sheen YH, Lee SW, An J, Kim MA, Han MY, Yon KD. Serum heavy metal levels are associated with asthma, allergic rhinitis, atopic dermatitis, allergic multimorbidity, and airflow obstruction. J Allergy Clin Immunol Pract. 2019;7(8):2912–2915.e2. doi: 10.1016/j.jaip.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Baldacci S, Maio S, Cerrai S, Sarno G, Baiz N, Simoni M, Annesi-Maesano I, Viegi G, HEALS Study Allergy and asthma: effects of the exposure to particulate matter and biological allergens. Respir Med. 2015;109(9):1089–1104. doi: 10.1016/j.rmed.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Leikauf GD, Kim SH, Jang AS. Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med. 2020;52(3):329–337. doi: 10.1038/s12276-020-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Georas S, Alexis N, Fritz P, Xia T, Williams MA, Horner E, Nel A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J Allergy Clin Immunol. 2016;138(2):386–396. doi: 10.1016/j.jaci.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Díaz-Robles LA, Fu JS, Vergara-Fernández A, Etcharren P, Schiappacasse LN, Reed GD, Reed GD, Silva MP. Health risks caused by short term exposure to ultrafine particles generated by residential wood combustion: a case study of Temuco, Chile. Environ Int. 2014;66:174–181. doi: 10.1016/j.envint.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Cereceda-Balic F, Toledo M, Vidal V, Guerrero F, Diaz-Robles LA, Petit-Breuilh X, Lapuerta M. Emission factors for PM 2.5, CO, CO 2, NO x, SO 2 and particle size distributions from the combustion of wood species using a new controlled combustion chamber 3CE. Sci Total Environ. 2017;584–585:901–910. doi: 10.1016/j.scitotenv.2017.01.136. [DOI] [PubMed] [Google Scholar]
- 39.Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res. 2014;129:11–19. doi: 10.1016/j.envres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weichenthal S, Bai L, Hatzopoulou M, Van Ryswyk K, Kwong JC, Jerrett M, Donkelaar A, Martin RV, Burnett RT, Lu H, Chen H. Long-term exposure to ambient ultrafine particles and respiratory disease incidence in in Toronto, Canada: a cohort study. Environ Health. 2017;16(1):64. doi: 10.1186/s12940-017-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clifford S, Mazaheri M, Salimi F, Ezz WN, Yeganeh B, Low-Choy S, Walker K, Mengersen K, Marks GB, Morawska L. Effects of exposure to ambient ultrafine particles on respiratory health and systemic inflammation in children. Environ Int. 2018;114:167–180. doi: 10.1016/j.envint.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Schaumann F, Frömke C, Dijkstra D, Alessandrini F, Windt H, Karg E, Müller M, Winkler C, Braun A, Koch A, Hohlfeld JM, Behrendt H, Schmid O, Koch W, Schulz H, Krug N. Effects of ultrafine particles on the allergic inflammation in the lung of asthmatics: results of a double-blinded randomized cross-over clinical pilot study. Part Fibre Toxicol. 2014;11:39. doi: 10.1186/s12989-014-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan S, Panacherry S. Asthma, environment and pollution: where the rubber hits the road. Indian J Pediatr. 2018;85(10):893–898. doi: 10.1007/s12098-018-2691-3. [DOI] [PubMed] [Google Scholar]
- 44.Government of Canada (2016) Human health risk assessment for ambient nitrogen dioxide. https://www.canada.ca/en/health-canada/services/publications/healthy-living/human-health-risk-assessment-ambient-nitrogen-dioxide.html. Accessed 25 Feb 2020
- 45.United States Environmental Protection Agency (2016) Integrated Science Assessment (ISA) for nitrogen dioxide: health criteria. https://www.epa.gov/isa/integrated-science-assessment-isa-nitrogen-dioxide-health-criteria. Accessed 25 Feb 2020
- 46.Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Effect of outdoor air pollution on asthma exacerbations in children and adults: systematic review and multilevel meta-analysis. PLoS ONE. 2017;12(3):e0174050. doi: 10.1371/journal.pone.0174050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassoun Y, James C, Bernstein DI. The effects of air pollution on the development of atopic disease. Clin Rev Allergy Immunol. 2019;57(3):403–414. doi: 10.1007/s12016-019-08730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cancado JE, Braga A, Pereira LA, Arbex MA, Saldiva PH, Santos Ude P. Clinical repercussions of exposure to atmospheric pollution. J Bras Pneumol. 2006;32(Suppl 2):S5–S11. doi: 10.1590/S1806-37132006000800003. [DOI] [PubMed] [Google Scholar]
- 49.Martins LC, Latorre Mdo R, Saldiva PH, Braga AL. Air pollution and emergency room visits due to chronic lower respiratory diseases in the elderly: an ecological time-series study in Sao Paulo. Brazil J Occup Environ Med. 2002;44(7):622–627. doi: 10.1097/00043764-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Arbex MA, Martins LC, de Oliveira RC, Pereira LA, Arbex FF, Cancado JE, Saldiva PHN, Braga ALF. Air pollution from biomass burning and asthma hospital admissions in a sugar cane plantation area in Brazil. J Epidemiol Community Health. 2007;61(5):395–400. doi: 10.1136/jech.2005.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arbex MA, Santos UP, Martins LC, Saldiva PH, Pereira LA, Braga AL. Air pollution and the respiratory system. J Bras Pneumol. 2012;38(5):643–655. doi: 10.1590/S1806-37132012000500015. [DOI] [PubMed] [Google Scholar]
- 52.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359(9304):386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Chen Q, Zheng X, Li Y, Han M, Liu T, Xiao J, Guo L, Zeng W, Zhang J, Ma W. Effects of ambient ozone concentrations with different averaging times on asthma exacerbations: a meta-analysis. Sci Total Environ. 2019;691:549–561. doi: 10.1016/j.scitotenv.2019.06.382. [DOI] [PubMed] [Google Scholar]
- 54.Esposito S, Tenconi R, Lelii M, Preti V, Nazzari E, Consolo S, Patria MF. Possible molecular mechanisms linking air pollution and asthma in children. BMC Pulm Med. 2014;14:31. doi: 10.1186/1471-2466-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esposito S, Galeone C, Lelii M, Longhi B, Ascolese B, Senatore L, Prada E, Montinaro V, Malerba S, Patria MF, Principi N. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulm Med. 2014;14:130. doi: 10.1186/1471-2466-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H, Tian Y, Xiang X, Li M, Wu Y, Cao Y, Juan J, Song J, Wu T, Hu Y. Association of short-term exposure to ambient carbon monoxide with hospital admissions in China. Sci Rep. 2018;8(1):13336. doi: 10.1038/s41598-018-31434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohara Y, Ohara T, Hashimoto K, Hosoya M. Exhaled carbon monoxide levels in infants and toddlers with episodic asthma. Fukushima J Med Sci. 2020;66(2):78–87. doi: 10.5387/fms.2019-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madl AK, Plummer LE, Carosino C, Pinkerton KE. Nanoparticles, lung injury, and the role of oxidant stress. Annu Rev Physiol. 2014;76:447–465. doi: 10.1146/annurev-physiol-030212-183735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Global Astma Network (2018) The Global Asthma Report 2018. http://www.globalasthmareport.org/Global%20Asthma%20Report%202018.pdf. Accessed 25 Feb 2020
- 60.Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ebmeier S, Thayabaran D, Braithwaite I, Bénamara C, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012) Lancet. 2017;390(10098):935–945. doi: 10.1016/S0140-6736(17)31448-4. [DOI] [PubMed] [Google Scholar]
- 62.D'Amato G, Vitale C, Molino A, Stanziola A, Sanduzzi A, Vatrella A, Mormile M, Lanza M, Calabrese G, Antonicelli L, D'Amato M. Asthma-related deaths. Multidiscip. Respir Med. 2016;11:37. doi: 10.1186/s40248-016-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veremchuk LV, Tsarouhas K, Vitkina TI, Mineeva EE, Gvozdenko TA, Antonyuk MV, Rakitskii VN, Sidletskaya KA, Tsatsakis AM, Golokhvast KS. Impact evaluation of environmental factors on respiratory function of asthma patients living in urban territory. Environ Pollut. 2018;235:489–496. doi: 10.1016/j.envpol.2017.12.122. [DOI] [PubMed] [Google Scholar]
- 64.Anenberg SC, Henze DK, Tinney V, Kinney PL, Raich W, Fann N, Malley CS, Roman H, Lamsal L, Duncan B, Martin RV, Donkelaar A, Brauer M, Doherty R, Jonson JE, Davila Y, Sudo K, Kuylenstierna JCI (2018) Estimates of the global burden of ambient PM2.5, ozone, and NO2 on asthma incidence and emergency room visits. Environ Health Perspect 126(10):107004. 10.1289/EHP3766 [DOI] [PMC free article] [PubMed]
- 65.Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Association of outdoor air pollution with the prevalence of asthma in children of Latin America and the Caribbean: a systematic review and meta-analysis. J Asthma. 2018;55(11):1174–1186. doi: 10.1080/02770903.2017.1402342. [DOI] [PubMed] [Google Scholar]
- 66.Watts J. Doctors blame air pollution for China's asthma increases. Lancet. 2006;368(9537):719–720. doi: 10.1016/S0140-6736(06)69267-2. [DOI] [PubMed] [Google Scholar]
- 67.Dong GH, Chen T, Liu MM, Wang D, Ma YN, Ren WH, Lee YL, Zhao Y-D, He Q-C. Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: northeast Chinese children health study. PLoS ONE. 2011;6(7):e22470. doi: 10.1371/journal.pone.0022470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Achakulwisut P, Brauer M, Hystad P, Anenberg SC. Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO2 pollution: estimates from global datasets. Lancet Planet Health. 2019;3(4):e166–e178. doi: 10.1016/S2542-5196(19)30046-4. [DOI] [PubMed] [Google Scholar]
- 69.Lee SW, Yon DK, James CC, Lee S, Koh HY, Sheen YH, Oh J-W, Han MY, Sugihara G. Short-term effects of multiple outdoor environmental factors on risk of asthma exacerbations: age-stratified time-series analysis. J Allergy Clin Immunol. 2019;144(6):1542–1550.e1. doi: 10.1016/j.jaci.2019.08.037. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, Hu J, Tan Z, Liu T, Zeng W, Li X, Huang C, Wang S, Huang Z, Ma W. Ambient carbon monoxide and increased risk of daily hospital outpatient visits for respiratory diseases in Dongguan, China. Sci Total Environ. 2019;668:254–260. doi: 10.1016/j.scitotenv.2019.02.333. [DOI] [PubMed] [Google Scholar]
- 71.Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res. 2011;111(3):418–424. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 72.Li MH, Fan LC, Mao B, Yang JW, Choi AMK, Cao WJ, Xu J-F. Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: a systematic review and meta-analysis. Chest. 2016;149(2):447–458. doi: 10.1378/chest.15-0513. [DOI] [PubMed] [Google Scholar]
- 73.Mascarenhas MD, Vieira LC, Lanzieri TM, Leal AP, Duarte AF. Hatch DL (2008) Anthropogenic air pollution and respiratory disease-related emergency room visits in Rio Branco, Brazil–September. J Bras Pneumol. 2005;34(1):42–46. doi: 10.1590/s1806-37132008000100008. [DOI] [PubMed] [Google Scholar]
- 74.Gruzieva O, Gehring U, Aalberse R, Agius R, Beelen R, Behrendt H, Bellander T, Birk M, Jongste JC, Fuertes E, Heinrich J, Hoek G, Klümper C, Koppelman G, Korek M, Krämer U, Lindley S, Mölter A, Simpson A, Standl M, Hage M, Berg A, Wijga A, Brunekreef B, Pershagen G. Meta-analysis of air pollution exposure association with allergic sensitization in European birth cohorts. J Allergy Clin Immunol. 2014;133(3):767–776.e7. doi: 10.1016/j.jaci.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 75.Molter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, Jongste J, Vocht F, Fuertes E, Gehring U, Gruzieva O, Heinrich J, Hoek G, Hoffmann B, Klümper C, Korek M, Kuhlbusch TAJ, Lindley S, Postma D, Tischer C, Wijga A, Pershagen G, Agius R. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J. 2015;45(3):610–624. doi: 10.1183/09031936.00083614. [DOI] [PubMed] [Google Scholar]
- 76.Khreis H, Cirach M, Mueller N, de Hoogh K, Hoek G, Nieuwenhuijsen MJ, Rojas-Rueda D (2019) Outdoor air pollution and the burden of childhood asthma across Europe. Eur Respir J 54(4):pii: 1802194. 10.1183/13993003.02194-2018 [DOI] [PubMed]
- 77.Gowers AM, Cullinan P, Ayres JG, Anderson HR, Strachan DP, Holgate ST, Mills IC, Maynard RL. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology. 2012;17(6):887–898. doi: 10.1111/j.1440-1843.2012.02195.x. [DOI] [PubMed] [Google Scholar]
- 78.Hutchinson JA, Vargo J, Milet M, French NHF, Billmire M, Johnson J, Hoshiko S. The San Diego 2007 wildfires and Medi-Cal emergency department presentations, inpatient hospitalizations, and outpatient visits: an observational study of smoke exposure periods and a bidirectional case-crossover analysis. PLoS Med. 2018;15(7):e1002601. doi: 10.1371/journal.pmed.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, Oldenwening M, Smit HA, Brunekreef B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181(6):596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 80.Alotaibi R, Bechle M, Marshall JD, Ramani T, Zietsman J, Nieuwenhuijsen MJ, Khreis H. Traffic related air pollution and the burden of childhood asthma in the contiguous United States in 2000 and 2010. Environ Int. 2019;127:858–867. doi: 10.1016/j.envint.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 81.Herbert C, Kumar RK. Ambient air pollution and asthma. Eur Respir J. 2017;49(5):1700230. doi: 10.1183/13993003.00230-2017. [DOI] [PubMed] [Google Scholar]
- 82.Fuertes E, Sunyer J, Gehring U, Porta D, Forastiere F, Cesaroni G, Vrijheid M, Guxens M, Annesi-Maesano I, Slama R, Maier D, Kogevinas M, Bousquet J, Chatzi L, Lertxundi A, Basterrechea M, Esplugues A, Ferrero A, Wright J, Mason D, McEachan R, Garcia-Aymerich J, Jacquemin B. Associations between air pollution and pediatric eczema, rhinoconjunctivitis and asthma: a meta-analysis of European birth cohorts. Environ Int. 2020;136:105474. doi: 10.1016/j.envint.2020.105474. [DOI] [PubMed] [Google Scholar]
- 83.Gehring U, Wijga AH, Koppelman GH, Vonk JM, Smit HÁ, Brunekreef B. Air pollution and the development of asthma from birth until young adulthood. Eur Respir J. 2020;56(1):2000147. doi: 10.1183/13993003.00147-2020. [DOI] [PubMed] [Google Scholar]
- 84.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31. doi: 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Perez L, Lurmann F, Wilson J, Pastor M, Brandt SJ, Künzli N, McConnell R. Near-roadway pollution and childhood asthma: implications for developing “win-win” compact urban development and clean vehicle strategies. Environ Health Perspect. 2012;120(11):1619–1626. doi: 10.1289/ehp.1104785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez L, Declercq C, Iniguez C, Aguilera I, Badaloni C, Ballester F, Bouland C, Chanel O, Cirarda FB, Forastiere F, Forsberg B, Haluza D, Hedlund B, Cambra K, Lacasaña M, Moshammer H, Otorepec P, Rodríguez-Barranco M, Medina S, Künzli N. Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network) Eur Respir J. 2013;42(3):594–605. doi: 10.1183/09031936.00031112. [DOI] [PubMed] [Google Scholar]
- 87.Rice MB, Rifas-Shiman SL, Litonjua AA, Gillman MW, Liebman N, Kloog I, Luttmann-Gibson H, Coull BA, Schwartz J, Koutrakis P, Oken E, Mittleman MA, Gold DR. Lifetime air pollution exposure and asthma in a pediatric birth cohort. J Allergy Clin Immunol. 2018;141(5):1932–1934.e7. doi: 10.1016/j.jaci.2017.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thurston GD, Balmes JR, Garcia E, Gilliland FD, Rice MB, Schikowski T, Van Winkle LS, Annesi-Maesano I, Burchard EG, Carlsten C, Harkema JR, Khreis H, Kleeberger SR, Kodavanti UP, London SJ, McConnell R, Peden DB, Pinkerton KE, Reibman J (2020) Outdoor air pollution and new-onset airway disease. An official American Thoracic Society Workshop Report. Ann Am Thorac Soc 17(4):387–398. 10.1513/AnnalsATS.202001-046ST [DOI] [PMC free article] [PubMed]
- 89.MacIntyre EA, Gehring U, Molter A, Fuertes E, Klümper C, Krämer U, Quass U, Hoffmann B, Gascon M, Brunekreef B, Koppelman GH, Beelen R, Hoek G, Birk M, Jongste JC, Smit HA, Cyrys J, Gruzieva O, Korek M, Bergström A, Agius RM, Vocht F, Simpson A, Porta D, Forastiere F, Badaloni C, Cesaroni G, Esplugues A, Fernández-Somoano A, Lerxundi A, Sunyer J, Cirach M, Nieuwenhuijsen MJ, Pershagen G, Heinrichcorresponding J. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect. 2014;122(1):107–113. doi: 10.1289/ehp.1306755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacquemin B, Siroux V, Sanchez M, Carsin AE, Schikowski T, Adam M, Bellisario V, Buschka A, Bono R, Brunekreef B, Cai Y, Cirach M, Clavel-Chapelon F, Declercq C, Marco R, Nazelle A, Ducret-Stich RE, Ferretti VV, Gerbase MW, Hardy R, Heinrich J, Janson C, Jarvis D, Kanaani ZA, Keidel D, Kuh D, Moual N, Nieuwenhuijsen MJ, Marcon A, Modig L, Pin I, Rochat T, Schindler C, Sugiri D, Stempfelet M, Temam S, Tsai M-Y, Varraso R, Vienneau D, Vierkötter A, Hansell AL, Krämer U, Probst-Hensch NM, Sunyer J, Künzli N, Kauffmann F. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE) Environ Health Perspect. 2015;123(6):613–621. doi: 10.1289/ehp.1408206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Global Initiative for Asthma (2020) 2020 GINA Report: global strategy for asthma management and prevention. https://ginasthma.org/gina-reports/. Access 25 Sept 2020
- 92.Künzli N, Bridevaux PO, Liu LJ, Garcia-Esteban R, Schindler C, Gerbase MW, Sunyer J, Keidel D, Rochat T, Swiss Cohort Study on Air Pollution and Lung Diseases in Adults Traffic-related air pollution correlates with adult-onset asthma among never-smokers. Thorax. 2009;64(8):664–870. doi: 10.1136/thx.2008.110031. [DOI] [PubMed] [Google Scholar]
- 93.Khreis H, de Hoogh K, Nieuwenhuijsen MJ. Full-chain health impact assessment of traffic-related air pollution and childhood asthma. Environ Int. 2018;114:365–375. doi: 10.1016/j.envint.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Cai Y, Zijlema WL, Doiron D, Blangiardo M, Burton PR, Fortier I, Gaye A, Gulliver J, Hoogh K, Hveem K, Mbatchou S, Morley DW, Stolk RP, Elliott P, Hansell A, Hodgson S. Ambient air pollution, traffic noise and adult asthma prevalence: a BioSHaRE approach. Eur Respir J. 2017;49(1):1502127. doi: 10.1183/13993003.02127-2015. [DOI] [PubMed] [Google Scholar]
- 95.United States Environmental Protection Agency (2018) Integrated Science Assessment (ISA) for particulate matter (first external review draft, Oct 2018). https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=341593. Access 25 Feb 2020
- 96.Tiotiu AI, Novakova P, Nedeva D, Chong-Neto HJ, Novakova S, Steiropoulos P, Kowal K. Impact of air pollution on asthma outcomes. Int J Environ Res Public Health. 2020;17(17):6212. doi: 10.3390/ijerph17176212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Havet A, Zerimech F, Sanchez M, Siroux V, Le Moual N, Brunekreef B, Stempfelet M, Künzli N, Jacquemin B, Matran R, Nadif R. Outdoor air pollution, exhaled 8-isoprostane and current asthma in adults: the EGEA study. Eur Respir J. 2018;51(4):1702036. doi: 10.1183/13993003.02036-2017. [DOI] [PubMed] [Google Scholar]
- 98.Jacquemin B, Schikowski T, Carsin AE, Hansell A, Krämer U, Sunyer J, Probst-Hensch N, Kauffmann F, Künzli N. The role of air pollution in adult-onset asthma: a review of the current evidence. Semin Respir Crit Care Med. 2012;33(6):606–619. doi: 10.1055/s-0032-1325191. [DOI] [PubMed] [Google Scholar]
- 99.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ (2014) Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med 190(8):914–921. 10.1164/rccm.201403-0525OC [DOI] [PMC free article] [PubMed]
- 100.Pope CA, 3rd, Lefler JS, Ezzati M, Higbee JD, Marshall JD, Kim SY, Bechle M, Gilliat KS, Vernon SE, Robinson AL, Burnett RT (2019) Mortality risk and fine particulate air pollution in a large, representative cohort of U.S. adults. Environ Health Perspect 127(7):77007. 10.1289/EHP4438 [DOI] [PMC free article] [PubMed]
- 101.Pope CA, 3rd, Coleman N, Pond ZA, Burnett RT. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res. 2020;183:108924. doi: 10.1016/j.envres.2019.108924. [DOI] [PubMed] [Google Scholar]
- 102.Bowatte G, Lodge CJ, Knibbs LD, Erbas B, Perret JL, Jalaludin B, Morgan GG, Bui DS, Giles GG, Hamilton GS, Wood-Baker R, Thomas P, Thompson BR, Matheson MC, Abramson MJ, Walters EH, Dharmage SC. Traffic related air pollution and development and persistence of asthma and low lung function. Environ Int. 2018;113:170–176. doi: 10.1016/j.envint.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 103.Havet A, Li Z, Zerimech F, Sanchez M, Siroux V, Le Moual N, Brunekreef B, Künzli N, Jacquemin B, Varraso R, Matran R, Nadif R. Does the oxidative stress play a role in the associations between outdoor air pollution and persistent asthma in adults? Findings from the EGEA study. Environ Health. 2019;18(1):90. doi: 10.1186/s12940-019-0532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rage E, Siroux V, Künzli N, Pin I, Kauffmann F. Air pollution and asthma severity in adults. Occup Environ Med. 2009;66(3):182–188. doi: 10.1136/oem.2007.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ścibor M, Malinowska-Cieślik M (2020) The association of exposure to PM10 with the quality of life in adult asthma patients. Int J Occup Med Environ Health 33(3):311-324. 10.13075/ijomeh.1896.01527 [DOI] [PubMed]
- 106.Garcia E, Berhane KT, Islam T, McConnell R, Urman R, Chen Z, Gilliland FD. Association of changes in air quality with incident asthma in children in California, 1993–2014. JAMA. 2019;321(19):1906–1915. doi: 10.1001/jama.2019.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friedman MS, Powell KE, Hutwagner L, Graham LM, Teague WG. Impact of changes in transportation and commuting behaviors during the 1996 Summer Olympic Games in Atlanta on air quality and childhood asthma. JAMA. 2001;285(7):897–905. doi: 10.1001/jama.285.7.897. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Wang W, Kan H, Xu X, Chen B. Air quality and outpatient visits for asthma in adults during the 2008 Summer Olympic Games in Beijing. Sci Total Environ. 2010;408(5):1226–1227. doi: 10.1016/j.scitotenv.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 109.Berman JD, Ebisu K (2020) Changes in U.S. air pollution during the COVID-19 pandemic. Sci Total Environ 739:139864. 10.1016/j.scitotenv.2020.139864 [DOI] [PMC free article] [PubMed]
- 110.Dutheil F, Navel V, Clinchamps M. The indirect benefit on respiratory health from the world’s effort to reduce transmission of SARS–CoV-2. Chest. 2020;158(2):467–468. doi: 10.1016/j.chest.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eguiluz-Gracia I, Tay TR, Hew M, Escribese MM, Barber D, O'Hehir RE, Torres MJ. Recent developments and highlights in biomarkers in allergic diseases and asthma. Allergy. 2018;73(12):2290–2305. doi: 10.1111/all.13628. [DOI] [PubMed] [Google Scholar]
- 112.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(17):2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 113.Sly PD, Cormier SA, Lomnicki S, Harding JN, Grimwood K. Environmentally persistent free radicals: linking air pollution and poor respiratory health? Am J Respir Crit Care Med. 2019;200(8):1062–1063. doi: 10.1164/rccm.201903-0675LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rao X, Zhong J, Brook RD, Rajagopalan S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid Redox Signal. 2018;28(9):797–818. doi: 10.1089/ars.2017.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hew KM, Walker AI, Kohli A, Garcia M, Syed A, McDonald-Hyman C, Noth EM, Mann JK, Pratt B, Balmes J, Hammond SK, Eisen EA, Nadeau KC. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy. 2015;45(1):238–248. doi: 10.1111/cea.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.MacNee W, Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur Respir J Suppl. 2003;40:47s–51s. doi: 10.1183/09031936.03.00403203. [DOI] [PubMed] [Google Scholar]
- 117.Cakmak S, Dales RE, Coates F (212) Does air pollution increase the effect of aeroallergens on hospitalization for asthma? J Allergy Clin Immunol 129(1):228–231. 10.1016/j.jaci.2011.09.025 [DOI] [PubMed]
- 118.Guilbert A, Cox B, Bruffaerts N, Hoebeke L, Packeu A, Hendrickx M, Cremer K, Bladt S, Brasseur O, Van Nieuwenhuyse A. Relationships between aeroallergen levels and hospital admissions for asthma in the Brussels-Capital Region: a daily time series analysis. Environ Health. 2018;17(1):35. doi: 10.1186/s12940-018-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alashkar Alhamwe B, Miethe S, Pogge von Strandmann E, Potaczek DP, Garn H. Epigenetic regulation of airway epithelium immune functions in asthma. Front Immunol. 2020;11:1747. doi: 10.3389/fimmu.2020.01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ji H, Biagini Myers JM, Brandt EB, Brokamp C, Ryan PH, Khurana Hershey GK. Air pollution, epigenetics, and asthma. Allergy Asthma Clin Immunol. 2016;12:51. doi: 10.1186/s13223-016-0159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]