Abstract
Background
Critically ill patients with COVID-19 may develop multiple organ dysfunction syndrome, including acute kidney injury (AKI). We report the incidence, risk factors, associations, and outcomes of AKI and renal replacement therapy (RRT) in critically ill COVID-19 patients.
Methods
We performed a retrospective cohort study of adult patients with COVID-19 diagnosis admitted to the intensive care unit (ICU) between March 2020 and May 2020. Multivariable logistic regression analysis was applied to identify risk factors for the development of AKI and use of RRT. The primary outcome was 60-day mortality after ICU admission.
Results
101 (50.2%) patients developed AKI (72% on the first day of invasive mechanical ventilation [IMV]), and thirty-four (17%) required RRT. Risk factors for AKI included higher baseline Cr (OR 2.50 [1.33–4.69], p = 0.005), diuretic use (OR 4.14 [1.27–13.49], p = 0.019), and IMV (OR 7.60 [1.37–42.05], p = 0.020). A higher C-reactive protein level was an additional risk factor for RRT (OR 2.12 [1.16–4.33], p = 0.023). Overall 60-day mortality was 14.4% {23.8% (n = 24) in the AKI group versus 5% (n = 5) in the non-AKI group (HR 2.79 [1.04–7.49], p = 0.040); and 35.3% (n = 12) in the RRT group versus 10.2% (n = 17) in the non-RRT group, respectively (HR 2.21 [1.01–4.85], p = 0.047)}.
Conclusions
AKI was common among critically ill COVID-19 patients and occurred early in association with IMV. One in 6 AKI patients received RRT and 1 in 3 patients treated with RRT died in hospital. These findings provide important prognostic information for clinicians caring for these patients.
Keywords: COVID-19, Severe acute respiratory syndrome coronavirus 2, Acute kidney injury, Dialysis, Intensive care unit, Continuous renal replacement therapy
Introduction
The first case of COVID-19 in Brazil was confirmed on February 26, 2020, and 6 months later, Brazil had more than 3,500,000 diagnosed cases and was the country with the second highest number of cases worldwide [1]. The clinical spectrum of COVID-19 ranges from asymptomatic disease to respiratory failure and multiple organ dysfunction syndrome [2], resulting in a large number of intensive care unit (ICU) admissions [3, 4]. Previous studies have shown that about 5% of all cases and up to 30% of hospitalized patients require ICU admission [5, 6].
Acute kidney injury (AKI) is a common clinical problem in critically ill patients, especially in those with acute lung injury receiving invasive mechanical ventilation (IMV) [7, 8]. Initial data showed a variable incidence of AKI in COVID-19 patients, ranging from 0.5 to 23% [2, 9], but more recent reports have shown even higher incidences, with rates of up to 36%, and need for renal replacement therapy (RRT) in 5.2% of patients [3, 10]. The development of AKI was associated with worse outcomes [2, 9, 11, 12]. The aim of our study was to describe the incidence, risk factors, and impact of AKI and RRT on clinical outcomes in COVID-19 patients admitted to the ICU.
Materials and Methods
We included adult patients (≥18 years old) with confirmed severe acute respiratory syndrome coronavirus 2 infection admitted to the ICU of Hospital Israelita Albert Einstein (HIAE), a private quaternary teaching hospital in Brazil, between March 04 and May 13, 2020. A laboratory-confirmed case of severe acute respiratory syndrome coronavirus 2 infection was defined by a positive RT-PCR assay for a specimen collected via nasopharyngeal swab. Patients with CKD on dialysis were excluded from the study.
Demographic and clinical variables included age, sex, chronic medical conditions, regular medications, reason for ICU admission, date of onset of symptoms, and drugs prescribed during hospitalization. Simplified Acute Physiology Score 3 (SAPS3), Sequential Organ Failure Assessment Score (SOFA), and Charlson Comorbidity Index were recorded on the first day of ICU. We also collected data on IMV, extracorporeal membrane oxygenation, vasopressors, and RRT requirement. Laboratory results were gathered at ICU admission.
Baseline Cr was defined as the average of all values observed between 8 and 365 days prior to hospitalization [13]. In the absence of previous serum Cr records, the lowest Cr level during hospitalization was considered [14]. The estimated glomerular filtration rate was calculated using the CKD-Epidemiology Collaboration Cr equation [15]. AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [14].
Individuals were initially treated according to the institutional protocol (appendix). Timing of dialysis initiation and the choice of modality were at the discretion of the nephrology team. Regularly at our center, critically ill patients with AKI are initiated on continuous venovenous hemodiafiltration (CVVHDF) at a prescribed dose of 30–35 mL/kg/h of effluent and with regional citrate anticoagulation [16]. The Prismaflex AN69 ST150 set was used with the Prismaflex control unit (Baxter, IL, USA) to perform CVVHDF. We evaluated the delivered dialysis dose, the filtration fraction, and the fluid balance during the first 7 days of therapy. We assessed the filter life span, and the reasons for changing the dialysis set throughout the CRRT time. The local Ethics Committee reviewed and approved this study (CAAE 30525320.0.0000.0071). All patients were unable to provide consent themselves, so consent was gained using the appropriate emergency consent mechanisms.
Outcomes
The primary outcome was 60-day mortality after ICU admission. After hospital discharge, the follow-up was made either by accessing the medical records or by telephone contact. The same strategy was applied to 28-day mortality. For all other secondary outcomes, follow-up was made until May 23, 2020 which included the following: Cr at hospital discharge, ICU mortality, in-hospital mortality, duration of mechanical ventilation in survivors, ICU length of stay, hospital length of stay, and dependence on RRT at hospital discharge.
Statistical Analysis
A convenience sample was considered for this analysis, with consecutive patients included until the latest follow-up. There were no missing data for any of the outcomes. Continuous variables are presented as medians (quartile 25%–quartile 75%) and categorical variables as number and percentages. Patients were divided in groups according to development of AKI (AKI vs. non-AKI) and treatment with RRT (RRT vs. non-RRT) until the latest follow-up.
Baseline and clinical characteristics of the patients were compared among the groups using Fisher exact tests and Wilcoxon rank-sum tests. Time until ICU and hospital discharge is presented as Kaplan-Meier plots. The association of AKI or RRT with ICU and hospital mortality was reported as odds ratio with 95% confidence interval from generalized linear modelling with binomial distribution. The association with ICU and hospital length of stay was assessed using the subdistribution hazard ratio derived from a Fine-Gray competing risk model with death before the event treated as competing risk. The association of Cr with hospital discharge and duration of ventilation in survivors was assessed as the median difference from a quantile regression with Τ = 0.50 and results estimated with bootstrap with 1,000 resamples. To account for potential confounders, additional analyses adjusted by SAPS3 and Charlson Comorbidity Index were performed.
Multivariable logistic regression model was used to identify factors independently associated with development of AKI and need of RRT. A list of candidate baseline predictors was determined a priori and included only variables with a known or suspected relationship with outcome. The multivariable model was constructed considering variables with a p > 0.05 in the univariable analysis and confirmed using a backward elimination technique and the leaps algorithm to perform a best subset selection including exhaustive search based on the Bayesian information criteria before undergoing a final assessment for clinical and biological plausibility. Missingness among continuous predictors was present in less than 5% of the patients; thus, these values were imputed using the median value. Subsequent sensitivity to missingness was performed using multiple imputations. The Cox proportional hazard model was used to analyze the effect of AKI and RRT on 28- and 60-day mortality. Time from ICU admission until death at 28- and 60-day was presented as Kaplan-Meier plots. All analyses were conducted in R v.3.6.3 (R Foundation) [17], and significance level was set at 0.05.
Results
A total of 207 patients with COVID-19 admitted to the ICU were evaluated. Six patients on chronic hemodialysis were excluded. Of the remaining 201 patients, 123 (61%) were males with a median age of 64 years (IQR, 52–80) Table 1.
Table 1.
Baseline characteristics of the patients according to development of AKI or need of RRT
| Overall (n = 201) | AKI (n = 101) | Non-AKI (n = 100) | p value | RRT (n = 34) | Non-RRT (n = 166) | p value | |
|---|---|---|---|---|---|---|---|
| Age, years | 64.0 (52.0–80.0) | 73.0 (56.0–84.0) | 60.0 (45.0–72.2) | <0.001 | 69.0 (57.2–82.0) | 64.0 (48.2–80.0) | 0.074 |
| Male gender, n (%) | 123 (61.2) | 67 (66.3) | 56 (56.0) | 0.174 | 27 (79.4) | 96 (57.8) | 0.031 |
| BMI, kg/m2,a | 28.2 (24.6–32.1) | 28.7 (25.4–33.2) | 27.1 (24.3–31.1) | 0.114 | 28.7 (26.1–32.4) | 27.9 (24.5–31.9) | 0.189 |
| SAPS3 | 49.0 (42.0–55.0) | 52.0 (45.8–57.0) | 43.0 (42.0–53.0) | <0.001 | 52.0 (49.0–57.0) | 46.0 (42.0–55.0) | 0.008 |
| SOFA | 2.0 (0.0–4.0) | 3.0 (1.0–6.0) | 1.0 (0.0–2.0) | <0.001 | 4.0 (2.0–8.0) | 1.0 (0.0–3.0) | <0.001 |
| Source of ICU admission, n (%) | |||||||
| Emergency room | 96 (47.8) | 50 (49.5) | 46 (46.0) | 0.688 | 18 (52.9) | 77 (46.4) | 0.031 |
| Ward | 77 (38.3) | 35 (34.7) | 42 (42.0) | 7 (20.6) | 70 (42.2) | ||
| Step-down unit | 15 (7.5) | 9 (8.9) | 6 (6.0) | 4 (11.8) | 11 (6.6) | ||
| Other hospital | 13 (6.5) | 7 (6.9) | 6 (6.0) | 5 (14.7) | 8 (4.8) | ||
| Coexisting disorders, n (%) | |||||||
| Charlson Comorbidity Index | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 0.0 (0.0–1.0) | <0.001 | 2.0 (1.0–3.0) | 1.0 (0.0–2.0) | <0.001 |
| Hypertension | 98 (48.8) | 59 (58.4) | 39 (39.0) | 0.009 | 21 (61.8) | 77 (46.4) | 0.148 |
| Diabetes | 64 (31.8) | 39 (38.6) | 25 (25.0) | 0.055 | 19 (55.9) | 45 (27.1) | 0.002 |
| Heart failure | 17 (8.5) | 13 (12.9) | 4 (4.0) | 0.045 | 9 (26.5) | 8 (4.8) | <0.001 |
| Pneumopathy | 19 (9.5) | 9 (8.9) | 10 (10.0) | 0.982 | 2 (5.9) | 17 (10.2) | 0.639 |
| Coronary artery disease | 16 (8.0) | 13 (12.9) | 3 (3.0) | 0.020 | 6 (17.6) | 10 (6.0) | 0.054 |
| Arrythmia | 25 (12.4) | 17 (16.8) | 8 (8.0) | 0.092 | 7 (20.6) | 17 (10.2) | 0.161 |
| Smoking | 6 (3.0) | 2 (2.0) | 4 (4.0) | 0.669 | 2 (5.9) | 4 (2.4) | 0.596 |
| Solid neoplasia | 19 (9.5) | 13 (12.9) | 6 (6.0) | 0.155 | 4 (11.8) | 15 (9.0) | 0.862 |
| Hematological neoplasia | 12 (6.0) | 7 (6.9) | 5 (5.0) | 0.780 | 3 (8.8) | 9 (5.4) | 0.715 |
| At ICU admission, n (%) | |||||||
| Respiratory failure | 139 (69.2) | 75 (74.3) | 64 (64.0) | 0.155 | 32 (94.1) | 107 (64.5) | 0.001 |
| Use of noninvasive ventilation | 122 (60.7) | 65 (64.4) | 57 (57.0) | 0.356 | 23 (67.6) | 99 (59.6) | 0.497 |
| Use of invasive ventilation | 87 (43.3) | 70 (69.3) | 17 (17.0) | <0.001 | 34 (100.0) | 53 (31.9) | <0.001 |
| Hours ICU admission to intubation | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.611 | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.398 |
| Use of vasopressor | 82 (40.8) | 66 (65.3) | 16 (16.0) | <0.001 | 33 (97.1) | 49 (29.5) | <0.001 |
| Use of ECMO | 2 (1.0) | 2 (2.0) | 0 (0.0) | 0.482 | 2 (5.9) | 0 (0.0) | 0.028 |
| Deep sedation | 69 (34.3) | 57 (56.4) | 12 (12.0) | <0.001 | 32 (94.1) | 37 (22.3) | <0.001 |
| Medications, n (%) | |||||||
| ACEi and/or ARB | 60 (29.9) | 34 (33.7) | 26 (26.0) | 0.302 | 11 (32.4) | 49 (29.5) | 0.902 |
| Antibiotics | 193 (96.0) | 100 (99.0) | 93 (93.0) | 0.069 | 34 (100.0) | 159 (95.8) | 0.480 |
| Steroids | 104 (53.3) | 64 (67.4) | 40 (40.0) | <0.001 | 19 (67.9) | 84 (50.6) | 0.137 |
| Lopinavir-ritonavir | 25 (12.4) | 18 (17.8) | 7 (7.0) | 0.035 | 9 (26.5) | 16 (9.6) | 0.016 |
| Hydroxychloroquine + azithromycin | 168 (84.0) | 84 (84.0) | 84 (84.0) | 0.999 | 30 (88.2) | 137 (83.0) | 0.620 |
| Tocilizumab | 9 (4.5) | 7 (6.9) | 2 (2.0) | 0.177 | 3 (8.8) | 6 (3.6) | 0.378 |
| Convalescent plasma | 32 (15.9) | 19 (18.8) | 13 (13.0) | 0.351 | 4 (11.8) | 27 (16.3) | 0.689 |
| Signs and symptoms, n (%) | |||||||
| Days symptoms to diagnosis | 4.0 (2.0–7.0) | 3.0 (1.0–6.0) | 5.0 (3.0–8.0) | 0.001 | 3.0 (1.0–7.0) | 4.0 (2.0–7.0) | 0.076 |
| Days symptoms to hospital admission | 6.0 (3.0–9.0) | 6.0 (3.0–7.0) | 7.0 (4.0–10.0) | 0.002 | 6.0 (4.5–7.0) | 6.0 (3.0–9.0) | 0.658 |
| Days symptoms to ICU admission | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 0.5 (0.0–1.0) | 0.303 | 0.0 (0.0–1.0) | 1.0 (0.0–2.0) | 0.155 |
| Rhinorrhea | 51 (25.4) | 14 (13.9) | 37 (37.0) | <0.001 | 5 (14.7) | 46 (27.7) | 0.171 |
| Odynophagia | 23 (11.4) | 6 (5.9) | 17 (17.0) | 0.025 | 2 (5.9) | 21 (12.7) | 0.405 |
| Anosmia | 10 (5.0) | 2 (2.0) | 8 (8.0) | 0.101 | 0 (0.0) | 10 (6.0) | 0.300 |
| Cough | 175 (87.1) | 85 (84.2) | 90 (90.0) | 0.306 | 28 (82.4) | 147 (88.6) | 0.477 |
| Shortness of breath | 173 (86.1) | 83 (82.2) | 90 (90.0) | 0.162 | 28 (82.4) | 144 (86.7) | 0.688 |
| Fever | 174 (86.6) | 86 (85.1) | 88 (88.0) | 0.700 | 29 (85.3) | 144 (86.7) | 0.999 |
| Diarrhea | 40 (19.9) | 20 (19.8) | 20 (20.0) | 0.999 | 5 (14.7) | 35 (21.1) | 0.541 |
| Organ support | |||||||
| Maximum dose of norepinephrine, µg/kg/min | 0.10 (0.06–0.20) | 0.12 (0.07–0.23) | 0.06 (0.04–0.10) | 0.011 | 0.15 (0.10–0.25) | 0.10 (0.05–0.15) | 0.002 |
| Use of epinephrine, n (%) | 10 (5.0) | 9 (8.9) | 1 (1.0) | 0.024 | 7 (20.6) | 3 (1.8) | <0.001 |
| Use of dobutamine, n (%) | 13 (6.4) | 11 (10.9) | 2 (2.0) | 0.023 | 6 (17.6) | 7 (4.2) | 0.012 |
| Use of recruitment maneuver, n (%) | 34 (16.9) | 30 (29.7) | 4 (4.0) | <0.001 | 18 (52.9) | 16 (9.6) | <0.001 |
| Use of prone positioning, n (%) | 10 (5.0) | 9 (8.9) | 1 (1.0) | 0.024 | 6 (17.6) | 4 (2.4) | 0.001 |
Data are presented as median (quartile 25%–quartile 75%) or n (%). Percentages may not total 100 because of rounding. ACEi, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; RRT, renal replacement therapy; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment.
aBMI is in kilograms divided by the square of the height in meters.
Baseline Cr was available prior to admission in 103 patients (52%). Baseline estimated glomerular filtration rate was lower in patients with AKI than in those without (78.71 [57.98–98.33] vs. 96.75 [83.82–116.31], p < 0.001). Incidence of proteinuria was higher in AKI patients (69.8 vs. 28.6%, p = 0.002) The results are presented in Table 2.
Table 2.
Laboratory tests results according to the development of AKI or the need for RRT
| Overall (n = 201) | AKI (n = 101) | Non-AKI (n = 100) | p value | RRT (n = 34) | Non-RRT (n = 167) | p value | |
|---|---|---|---|---|---|---|---|
| Kidney function | |||||||
| Baseline Cr, mg/dL | 0.85 (0.7–1) | 0.90 (0.75–1.20) | 0.80 (0.68–0.90) | <0.001 | 1.02 (0.89–1.34) | 0.80 (0.66–0.93) | <0.001 |
| Baseline eGFR, mL/min/1.73 m2 | 89.9 (72.53–105.92) | 78.71 (57.98–98.33) | 96.75 (83.82–116.31) | <0.001 | 70.43 (48.64–86.03) | 93.19 (76.58–109.98) | <0.001 |
| Cr at hospital admission, mg/dL | 0.98 (0.8–1.25) | 1.20 (0.90–1.50) | 0.86 (0.75–1.00) | <0.001 | 1.33 (1.10–1.62) | 0.91 (0.78–1.13) | <0.001 |
| Cr at ICU admission, mg/dL | 0.98 (0.78–1.28) | 1.27 (0.93–1.64) | 0.85 (0.70–1.00) | <0.001 | 1.64 (1.33–2.27) | 0.90 (0.75–1.17) | <0.001 |
| Highest Cr during ICU admission, mg/dL | 1.16 (0.88–1.8) | 1.80 (1.34–2.90) | 0.90 (0.78–1.07) | <0.001 | 3.31 (2.44–4.63) | 1.05 (0.85–1.36) | <0.001 |
| Urine change, n (%) | 49 (24.4) | 35 (81.4) | 14 (50.0) | 0.011 | 11 (84.6) | 38 (65.5) | 0.311 |
| Hematuria | 35 (17.4) | 25 (58.1) | 10 (35.7) | 0.109 | 9 (69.2) | 26 (44.8) | 0.199 |
| Leukocyturia | 26 (12.9) | 20 (46.5) | 6 (21.4) | 0.058 | 7 (53.8) | 19 (32.8) | 0.268 |
| Proteinuria | 38 (18.9) | 30 (69.8) | 8 (28.6) | 0.002 | 11 (84.6) | 27 (46.6) | 0.029 |
| Laboratory test at ICU admission | |||||||
| Hemoglobin, g/dL | 12.9 (11.5–14) | 12.5 (11.3–13.9) | 13.0 (11.9–14.3) | 0.266 | 11.8 (10.8–13.8) | 12.9 (11.7–14.1) | 0.129 |
| White blood cell count, cells/mm3 | 6,815 (4,730–8,782.5) | 7,210 (4,910–9,960) | 6,590 (4,380–8,300) | 0.14 | 7,580 (5,580–9,960) | 6,670 (4,625–8,455) | 0.141 |
| Lymphocytes, cells/mm3 | 873.5 (627.5–1,255) | 828 (597–1,161) | 945 (691–1,264) | 0.074 | 826 (498–1,040) | 910 (689–1,257) | 0.075 |
| Lymphopenia, n (%)* | 118 (58.7) | 67 (69.1) | 51 (53.7) | 0.041 | 24 (72.7) | 94 (56.2) | 0.197 |
| Platelets, ×103 cells/mm3 | 179 (150–237) | 172 (134–235) | 185 (159–239) | 0.052 | 183 (146–237) | 177 (151–239) | 0.701 |
| D-dimer, ng/mL | 824 (488–1,245.75) | 876.5 (557.5–1,592.0) | 685.0 (420.5–1,136.0) | 0.009 | 1,359.0 (838.0–2,236.0) | 688.0 (451.5–1,136.0) | <0.001 |
| Ferritin | 947 (565.75–1,800.75) | 1,091 (616–2,080) | 869 (544–1,696) | 0.435 | 1,803 (1,016–3,577) | 884 (560–1,625) | 0.085 |
| Lactate dehydrogenase | 346 (287–442) | 354.0 (292.0–465.0) | 341.0 (287.0–424.0) | 0.225 | 376.0 (326.0–495.0) | 340.5 (275.8–424.5) | 0.043 |
| CRP, mg/dL | 97.2 (52.2–158) | 104.1 (63.6–194.0) | 83.7 (40.4–143.2) | 0.012 | 168.8 (107.0–230.5) | 86.3 (49.9–142.5) | <0.001 |
| Pro-calcitonin | 0.22 (0.11–0.48) | 0.29 (0.14–0.76) | 0.15 (0.07–0.22) | 0.02 | 0.55 (0.23–1.70) | 0.17 (0.08–0.28) | 0.006 |
| Cr phosphokinase | 78 (49–161) | 86.0 (49.5–207.0) | 69.0 (49.0–130.8) | 0.414 | 86.0 (66.0–288.0) | 76.0 (46.0–142.0) | 0.307 |
| Sodium, mEq/L | 138 (135–140) | 138 (135–140) | 138 (135–141) | 0.255 | 137 (135–140) | 138 (135–140) | 0.718 |
| Potassium, mEq/L | 4.05 (3.8–4.4) | 4.2 (3.8–4.5) | 4.0 (3.8–4.3) | 0.046 | 4.0 (3.6–4.4) | 4.1 (3.8–4.4) | 0.786 |
| Ionized calcium | 1.14 (1.1–1.18) | 1.14 (1.10–1.18) | 1.16 (1.12–1.19) | 0.143 | 1.15 (1.10–1.18) | 1.14 (1.10–1.18) | 0.734 |
| Phosphate, mEq/L | 3.5 (2.9–4.2) | 3.7 (3.0–4.4) | 3.4 (2.8–3.9) | 0.127 | 4.2 (3.7–5.0) | 3.3 (2.6–3.8) | <0.001 |
| Magnesium, mEq/L | 1.7 (1.5–1.8) | 1.6 (1.5–1.8) | 1.7 (1.6–1.9) | 0.002 | 1.6 (1.5–1.8) | 1.7 (1.6–1.8) | 0.294 |
| Urea, mg/dL | 30 (22–43.5) | 39.0 (26.0–65.0) | 27.0 (20.0–34.5) | <0.001 | 44.0 (33.0–68.0) | 28.0 (22.0–40.0) | <0.001 |
| Aspartate transaminase, U/L | 34.5 (25.25–51) | 44.0 (28.0–60.0) | 33.0 (24.0–46.0) | 0.081 | 61.0 (44.0–69.0) | 31.5 (25.0–46.8) | <0.001 |
| Alanine transaminase, U/L | 30.5 (22–48) | 31.0 (23.0–45.0) | 29.0 (18.0–55.0) | 0.924 | 37.0 (23.5–49.0) | 28.5 (22.0–47.2) | 0.274 |
| Bilirubin, mg/dL | 0.4 (0.2–0.5) | 0.4 (0.3–0.6) | 0.3 (0.2–0.5) | 0.014 | 0.5 (0.4–0.6) | 0.3 (0.2–0.5) | 0.002 |
| Interleukin-6 | 127.1 (55–356.8) | 226.9 (81.0–776.0) | 89.0 (28.6–140.8) | 0.005 | 776.0 (335.9–981.3) | 105.6 (35.6–160.8) | <0.001 |
| Arterial blood gas | |||||||
| pH | 7.41 (7.37–7.45) | 7.40 (7.35–7.44) | 7.43 (7.39–7.46) | 0.001 | 7.37 (7.32–7.42) | 7.42 (7.38–7.46) | <0.001 |
| PaO2, mm Hg | 70.2 (38.2–108.7) | 73.0 (41.2–115.5) | 58.9 (37.1–101.2) | 0.252 | 80.9 (42.6–124.5) | 68.1 (37.9–101.8) | 0.21 |
| PaCO2, mm Hg | 38.25 (33.7–44.2) | 39.4 (34.2–44.6) | 36.8 (33.0–43.6) | 0.143 | 40.0 (37.3–44.7) | 37.6 (32.8–43.5) | 0.028 |
| HCO3, mEq/L | 23.9 (22.1–25.8) | 23.6 (21.6–25.6) | 24.6 (22.9–26.2) | 0.059 | 23.3 (21.2–24.7) | 24.4 (22.2–25.8) | 0.068 |
| Base excess, mEq/L | 0.2 (−1.7 to 1.6) | –0.2 (−2.8 to 1.0) | 0.6 (−0.6 to 1.8) | 0.002 | –0.9 (−4.2 to 0.2) | 0.4 (−1.5 to 1.7) | 0.002 |
| Lactate, mg/dL | 12.0 (10–16) | 12.0 (10.0–16.0) | 12.0 (9.0–16.0) | 0.795 | 12.0 (10.0–12.8) | 13.0 (9.8–17.0) | 0.288 |
AKI, acute kidney injury; RRT, renal replacement therapy; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; CRP, C-reactive protein.
Lymphocyte count <1,000 cells/mm3.
AKI Characteristics
AKI was diagnosed in 50.2% (n = 101) of all patients, and the median age was 73 years (IQR, 56.0–84.0). Forty-three (42.6%) patients were stratified as stage 1; 19 (18.8%), stage 2; and 39 (38.6%), stage 3 AKI. Their processes of care are shown in Table 3.
Table 3.
Nephrology processes of care
| AKI (n = 101) | Non-AKI (n = 100) | p value | RRT (n = 34) | Non-RRT (n = 167) | p value | |
|---|---|---|---|---|---|---|
| AKI KDIGO stage, n | ||||||
| (%) 1 | 43 (42.6) | 0 (0.0) | 43 (64.2) | <0.001 | ||
| 2 | 19 (18.8) | − | − | 0 (0.0) | 19 (28.3) | |
| 3 | 39 (38.6) | − | − | 34 (100) | 5 (7.5) | |
| Nephrology process of care | ||||||
| Days from ICU admission to nephrology | 3.0 (2.0–7.0) | − | − | 3.0 (3.0–7.0) | 2.0 (2.0–9.5) | 0.427 |
| Days from ICU admission to RRT | − | − | − | 3.5 (3.0–7.0) | − | − |
| Days from IMV to RRT | − | − | − | 3.0 (2.0–5.75) | − | − |
| Days from AKI to RRT | − | − | − | 2.0 (1.0–5.0) | − | − |
| Days from nephrology to RRT | − | − | − | 0.0 (0.0–0.0) | − | − |
| CVVHDF modality, n (%) | − | − | − | 33 (97.0) | − | − |
| Change of modality during treatment, n (%) | − | − | − | 14 (53.8) | − | − |
| Days in the ICU using RRT | − | − | − | 15.0 (10.0–24.0) | − | − |
| Days in the hospital using RRT | − | − | − | 22.0 (10.0–29.0) | − | − |
Data are presented as median (quartile 25%–quartile 75%) or n (%). Percentages may not total 100 because of rounding. AKI, acute kidney injury; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; RRT, renal replacement therapy; IMV, invasive mechanical ventilation; CVVHDF, continuous venovenous hemodiafiltration.
After adjustment in a multivariable analysis, the development of AKI was independently associated with diuretic use, IMV, and higher baseline Cr (Table 4; online suppl. Tables 1–3; for all online suppl. material, see www.karger.com/doi/10.1159/000513425). AKI was developed in 80% (70/87) of the patients who required IMV. The median time between the beginning of IMV and the diagnosis of AKI was 1 day (IQR, 0–1). Seventy-two percent of patients were diagnosed with AKI during the first day of IMV.
Table 4.
Multivariable model after multiple imputation for AKI
| Odds ratio (95% CI) | p value | |
|---|---|---|
| Baseline and severity of illness | ||
| Age | 0.88 (0.43–1.81) | 0.734 |
| SAPS3 | 1.67 (0.71–3.92) | 0.237 |
| Comorbidities | ||
| Charlson Comorbidity Index | 1.24 (0.76–2.00) | 0.387 |
| Hypertension | 1.57 (0.66–3.70) | 0.303 |
| Drugs and support at ICU admission | ||
| Use of lopinavir-ritonavir | 1.99 (0.55–7.21) | 0.290 |
| Use of invasive ventilation | 7.60 (1.37–42.05) | 0.020 |
| Use of vasopressor | 0.58 (0.12–2.73) | 0.490 |
| Use of steroids | 1.41 (0.61–3.23) | 0.420 |
| Use of diuretic | 4.14 (1.27–13.49) | 0.019 |
| Laboratory tests at ICU admission | ||
| Lymphopenia | 1.20 (0.51–2.82) | 0.667 |
| Baseline Cr | 2.50 (1.33–4.69) | 0.005 |
| D-dimer | 1.22 (0.70–2.14) | 0.471 |
| CRP | 0.78 (0.49–1.23) | 0.280 |
CI, confidence interval; AKI, acute kidney injury; SAPS, Simplified Acute Physiology Score; ICU, intensive care unit; CRP, C-reactive protein.
Among survivors, patients with AKI remained on IMV longer (10.5 [6.5–16.8] vs. 6 [6–9] days; p < 0.001). The clinical outcomes according to the development of AKI are shown in Table 5. In online suppl. Figures 1 and 2, time until hospital and ICU discharge are presented as Kaplan-Meier curves, respectively.
Table 5.
Clinical outcomes according to the development of AKI or the need for RRT
| AKI (n = 101) | Non-AKI (n = 100) | Unadjusted effect estimate (95% CI) | p value | Adjusted effect estimate (95% CI)* | p value | |
|---|---|---|---|---|---|---|
| Renal outcomes | ||||||
| Cr at discharge, mg/dL | 1.00 (0.87–1.46) | 0.90 (0.72–1.00) | 0.15 (−0.05 to 0.34)a | 0.139 | 0.73 (0.22–1.24)a | 0.037 |
| Clinical outcomes | ||||||
| ICU mortality, n (%) | 21 (20.8) | 5 (5.0) | 5.00 (1.93–15.50)b | 0.002 | 2.81 (0.93–9.97)b | 0.081 |
| Hospital mortality, n (%) | 22 (21.8) | 5 (5.0) | 5.29 (2.06–16.37)b | 0.001 | 3.06 (1.01–10.94)b | 0.059 |
| 28-day mortality, n (%) | 18 (17.8) | 5 (5.0) | 3.72 (1.38–10.04)c | 0.009 | 1.72 (0.62–4.78)c | 0.301 |
| 60-day mortality, n (%) | 24 (23.8) | 5 (5.0) | 5.11 (1.95–13.40)c | <0.001 | 2.79 (1.04–7.49)c | 0.04 |
| Duration of ventilation in survivors, days | 10.5 (6.5–16.8) | 6.0 (3.0–9.0) | 5.22 (2.18–8.26)a | <0.001 | 6.52 (1.60–11.44)a | 0.009 |
| ICU length of stay, days | 13.5 (5.2–25.0) | 5.0 (3.0–8.0) | 0.36 (0.26–0.51)d | <0.001 | 0.44 (0.31–0.62)d | <0.001 |
| Hospital length of stay, days | 18.0 (12.8–32.2) | 10.0 (6.5–14.0) | 0.31 (0.22–0.44)d | <0.001 | 0.38 (0.27–0.55)d | <0.001 |
| RRT (n = 34) | Non-RRT (n = 166) | Unadjusted effect estimate (95% CI) | p value | Adjusted effect estimate (95% CI)* | p value | |
|---|---|---|---|---|---|---|
| Renal outcomes | ||||||
| Cr at discharge, mg/dL | 3.50 (2.00–4.34) | 0.90 (0.77–1.06) | 2.53 (0.94–4.11)a | 0.002 | 2.53 (1.02–4.05)a | 0.001 |
| RRT at hospital discharge | 1/9 (11.1) | − | − | − | − | − |
| Clinical outcomes | ||||||
| ICU mortality, n (%) | 12 (35.3) | 14 (8.4) | 5.92 (2.41–14.54)b | <0.001 | 4.39 (1.56–12.49)b | 0.005 |
| Hospital mortality, n (%) | 12 (35.3) | 15 (9.0) | 5.49 (2.25–13.32)b | <0.001 | 3.98 (1.42–11.15)b | 0.008 |
| 28-day mortality, n (%) | 7 (20.6) | 16 (9.6) | 2.27 (0.93–5.51)c | 0.071 | 1.11 (0.41–2.96)c | 0.836 |
| 60-day mortality, n (%) | 12 (35.3) | 17 (10.2) | 3.75 (1.79–7.85)c | <0.001 | 2.21 (1.01–4.85)c | 0.047 |
| Duration of ventilation in survivors, days | 17.0 (11.8–36.8) | 6.0 (4.5–11.0) | 12.78 (2.72–22.83)a | 0.012 | 12.91 (6.67–19.15)a | <0.001 |
| ICU length of stay, days | 29.0 (19.5–37.0) | 6.0 (3.0–11.0) | 0.23 (0.15–0.35)d | <0.001 | 0.26 (0.17–0.40)d | <0.001 |
| Hospital length of stay, days | 33.0 (30.0–40.0) | 12.0 (8.0–17.0) | 0.20 (0.12–0.35)d | <0.001 | 0.20 (0.12–0.35)d | <0.001 |
Data are presented as median (quartile 25%–quartile 75%) or n (%). Percentages may not total 100 because of rounding. CI, confidence interval; AKI, acute kidney injury; ICU, intensive care unit; RRT, renal replacement therapy.
All models are adjusted by SAPS3 and Charlson Comorbidity Index.
Effect estimate is median difference from a quantile regression with T = 0.50 and results estimated with bootstrap with 1,000 resamples.
Effect estimate is odds ratio from a generalized linear model with a binomial distribution.
Effect estimate is hazard ratio from a Cox proportional hazard model.
Effect estimate is subdistribution hazard ratio from a Fine-Gray competing risk model.
Dialysis Characteristics
Thirty-four percent of the 101 AKI patients received RRT. After adjustment on multivariable analysis, higher C-reactive protein (CRP) and baseline Cr were associated with RRT requirement (Table 6; online suppl. Tables 4–6). CVVHDF was the initial modality used in 97% of patients, who remained on RRT for a median of 15 days (IQR 10–24) during ICU stay (Table 3). The delivered dialysis dose of CVVHDF and the filtration fraction during the first 7 days of therapy were 36.7 mL/kg/h (32.2–37.9) and 21.7% (18.8–23.1), respectively. A simple analysis showed no significant difference in the delivered dialysis dose between the survivors and those who died: 36.8 (32.1–38.0) and 35.0 (33.7–37.7) mL/kg/h, respectively, p = 0.710. The median of water balance in the first 7 days of CRRT showed no significant difference between those who died and the survivors, 1.99 and 2.28 L, respectively, p = 0.488. The filter life span was 80.0 h (47.6–94.0). 161 filters were used. The main reasons for stopping the filter circuit were as follows: scheduled change (n = 78, 48.4%), early filter coagulation (n = 36, 22.4%), change of dialysis modality or recovery of kidney function (n = 25, 15.5%), technical problems (n = 7, 4.3%), for tests or procedures, (n = 5, 3.1%), and death or palliative care (n = 10, 6.2%).
Table 6.
Multivariable model after multiple imputation for RRT
| Odds ratio (95% CI) | p value | |
|---|---|---|
| Baseline and severity of illness SAPS3 | 1.13 (0.57–2.23) | 0.730 |
| Comorbidities | ||
| Charlson Comorbidity Index | 1.17 (0.67–2.04) | 0.574 |
| Drugs and support at ICU admission | ||
| Use of lopinavir-ritonavir | 1.87 (0.49–7.09) | 0.357 |
| Use of vasopressor | 55.20 (4.52–674.59) | 0.002 |
| Use of diuretics | 1.88 (0.25–14.00) | 0.536 |
| Laboratory tests at ICU admission | ||
| Cr | 2.90 (1.56–5.38) | 0.001 |
| D-dimer | 1.42 (0.94–2.14) | 0.099 |
| CRP | 1.96 (1.04–3.70) | 0.037 |
RRT, renal replacement therapy; CI, confidence interval; SAPS, Simplified Acute Physiology Score; ICU, intensive care unit; CRP, C-reactive protein.
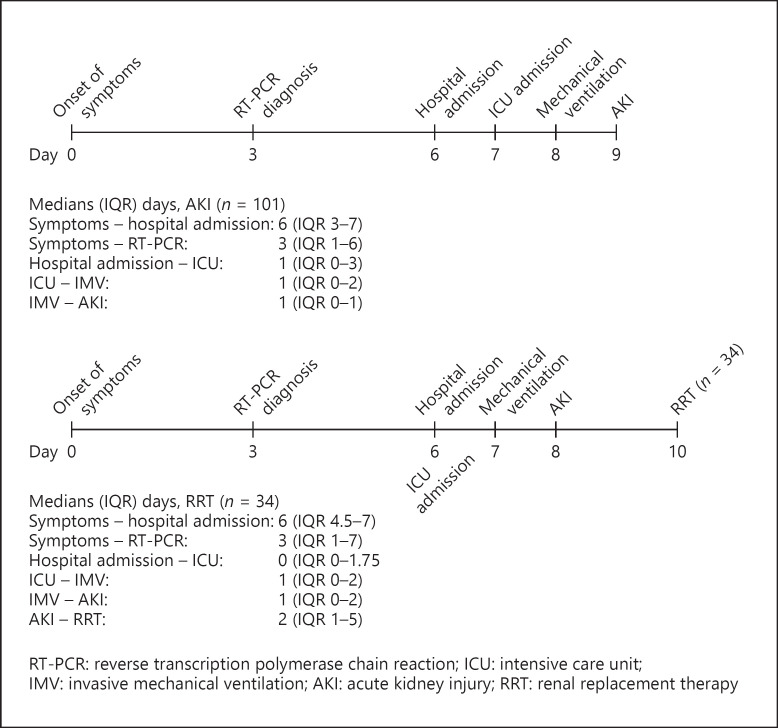
All patients receiving RRT were on IMV. Additional clinical outcomes according to the use of RRT are shown in Table 5. Figure 1 shows the time from onset of symptoms to AKI and RRT.
Fig. 1.
AKI and RRT timelines: the time from onset of symptoms to AKI or RRT.
Mortality
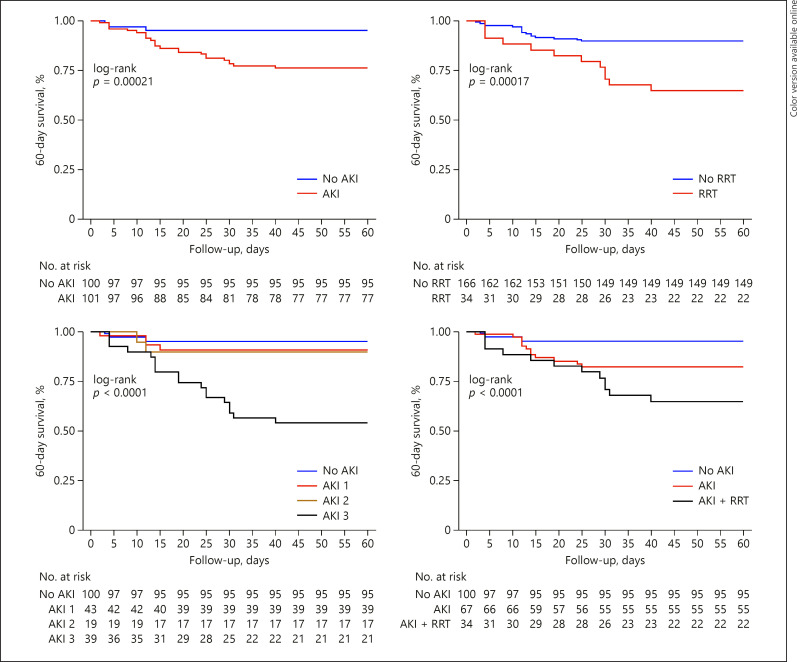
Overall 60-day mortality rate was 14.4% (n = 29). For AKI patients, the rate was 23.8% (n = 24) versus 5% (n = 5) for the non-AKI group (HR 2.79 [1.04–7.49], p = 0.040). The 60-day mortality rate in the RRT group was 35.3% (n = 12) versus 10.2% (n = 17) in the non-RRT group (HR 2.21 [1.01–4.85], p = 0.047). Survival curves are shown in Figure 2 and online suppl. Figure 3. The main causes of death were respiratory failure 51.7% (n = 15), shock 17.2% (n = 5), cardiovascular disease 17.2% (n = 5), and sepsis 13.8% (n = 4).
Fig. 2.
Kaplan-Meier curve showing 60-day survival. AKI, acute kidney injury; RRT, renal replacement therapy.
Discussion
Key Findings
In ICU patients with COVID-19, AKI occurred in half of cases and was associated with baseline serum Cr level, use of IMV, and use of diuretics. In patients with more severe AKI (stage 3D), baseline serum Cr levels and higher blood levels of CRP at ICU admission were associated with requiring RRT. In the AKI group, the duration of IMV was prolonged, and the length of stay in ICU and in hospital was longer. AKI and RRT were independent risk factors for 60-day mortality.
Relationship to Previous Findings
AKI in the context of COVID-19 has been subject to several previous studies. Cheng et al. [9] found an AKI rate of 5.1%. Lim et al. [18] reported an AKI incidence of 18.3% in hospitalized patients; among these, 37.5% were classified as stage 1. Hirsch et al. [3] showed that the incidence was even higher (36.6%), and the majority was in stage 1 (46.5%). Our study exclusively enrolled patients in the ICU, and the incidence of AKI was 50%. About 40% of these developed the mildest form (stage 1 AKI). Another initial small report of only 21 ICU patients demonstrated that 19% had AKI [11]. Recently, Gupta et al. [19] observed an incidence rate of 42.8% (restricted to stage 2 and 3) in 2,215 patients with COVID-19 admitted to ICU.
COVID-19 patients may also present with hematuria and proteinuria, suggesting inflammation and glomerular endothelial damage as an additional event in the genesis of AKI [12, 20, 21]. Our study shows that patients who have AKI also had a higher incidence of proteinuria. However, few patients had urinalysis available at the ICU admission and many had conditions associated with proteinuria (diabetes, hypertension, and obesity).
In the current study, 72% of patients were diagnosed with AKI in the first 24 h of mechanical ventilation and IMV increased the risk of having AKI by almost 8-fold. This link has also been observed elsewhere [3]. In a cohort that evaluated only patients on mechanical ventilation, AKI occurred in 75% of patients [22]. In our study, AKI developed in 80% of mechanically ventilated patients. In a systematic review and meta-analysis, van den Akker et al. [23] showed that IMV increased the odds for AKI by 3 times. Despite the difficulty of dissociating the effects of the lung infectious/inflammatory disease on kidney function, several studies suggest that IMV may induce or worsen AKI [7, 24] and several mechanisms have been proposed to explain this association [25, 26].
In our study, the use of a loop diuretic was associated with AKI. Studies using diuretics, especially furosemide, in patients at risk for or with overt AKI have presented mixed results [27, 28]. In the current study, we could not ascertain the cause and effect relationship between the use of diuretic and AKI.
Higher baseline Cr was associated with AKI in this analysis. Multiple mechanisms present in CKD make critically ill patients more prone to AKI, including inflammation, oxidative stress, and changes in microcirculation [29]. A postmortem histopathology study showed mild to severe arterionephrosclerosis, diabetic nephropathy, and features suggestive of acute tubular injury in a case series of fatal COVID-19 infections. Furthermore, some of these patients had AKI during hospital admission [30]. These findings suggest that acute injury-on-CKD may also be connected in patients with COVID-19.
Seventeen percent of overall patients required RRT. RRT was applied in 34% of patients with AKI and in 39% of those on IMV. According to Hirsch et al. [3], 5.2% of hospitalized patients with COVID-19 received RRT. Among patients with AKI, 14.6% underwent dialysis and, of those on dialysis, 96.8% were on ventilators [4]. Cummings et al. [31] analyzed 257 critically ill patients with COVID-19 and reported 31% on RRT. In the study by Gupta et al. [19], the need for dialysis was 20.1%. Fominskiy et al. [22] evaluated patients with COVID-19 on mechanical ventilation and showed that 17.7% of patients required dialysis. Thus, AKI stage 3D seems to be common in patients with COVID-19, especially in those on ventilatory support.
AKI could be a consequence of uncontrolled inflammation in COVID-19 patients [18, 22]. CRP blood level at ICU admission was an independent risk factor for dialysis-requiring AKI in our cohort. Thus, high CRP levels could identify patients more prone to organ dysfunction, including kidney failure. High CRP level was associated with AKI in other diseases as well [32, 33, 34].
AKI and RRT mortality rates in our analysis were lower compared to other studies [19, 25, 35]. Differences in admission policies, length of follow-up, learnings from the experience of the first centers to suffer from COVID-19 outbreaks, and differences in population or in the prevalence of comorbidities make these comparisons difficult. In our cohort of patients, AKI and RRT were independent risk for 60-day mortality. Two meta-analysis also showed AKI was associated with increased risk of mortality in COVID-19 patients [35, 36]. On the other hand, other studies have not shown AKI as a risk factor for death in COVID-19 [31, 37]. Like other diseases, AKI in COVID-19 patients seems to be associated with adverse outcomes and its awareness must be highlighted.
Study Implications
Our data confirm that AKI is a concern in patients with COVID-19 admitted to the ICU. Moreover, AKI develops temporally after the onset of IMV and its most severe stage is associated with baseline kidney dysfunction and signs associated with inflammation such as increased level of CRP. Finally, our findings provide important prognostic information regarding survival, time in ICU, time in hospital, and recovery of renal function in these patients.
Strengths and Limitations
To the best of our knowledge, this is the first report on AKI in critically ill patients from Brazil, currently one of the epicenters of the COVID-19 pandemic. It was possible to track the clinical course of the COVID-19 from the beginning of the first symptoms to the onset of RRT. During the follow-up and after the mortality analysis, we were able to verify a definitive primary outcome, 60-day mortality, in 100% of the events of our cohort and report on renal recovery.
Limitations exist as this is a single-center study and could include selection bias and limited external validity. However, it brings some useful information for professionals committed to the treatment of patients with COVID-19. ICU admission and discharge policies may differ between centers. However, the ICU multidisciplinary team developed a protocol comprising well-defined criteria for ICU admission. In 48% of the cases, we did not have a baseline Cr value and using the lowest value during hospitalization may have overestimated the incidence of AKI [13]. There is some controversy in using baseline Cr (independent variable) as a risk factor for AKI (dependent variable) since the definition of AKI requires this variable. However, multiple previous studies have assessed the role of pre-illness Cr as a predictor of AKI [38, 39, 40, 41, 42]. Finally, because this is an observational study, no cause and effect relationship can be inferred.
In conclusion, we found that in ICU-admitted COVID-19 patients, the occurrence of AKI was common and temporally associated with IMV. Patients with high levels of CRP, perhaps presenting a greater inflammatory state, presented with the most severe form of AKI. AKI and RRT were independent risk factors for 60-day mortality.
Statement of Ethics
A local Ethics Committee reviewed and approved this study (CAAE 30525320.0.0000.0071). All procedures were performed in accordance with the World Medical Association's Declaration of Helsinki. Informed consent was obtained from all patients using the appropriate emergency consent mechanisms.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Funding Sources
The authors did not receive any funding.
Author Contributions
M.S.D. designed and coordinated the study. M.P.D., F.R.T.C., and B.G.B. collected data. A.S.N. did statistical analysis. M.P.D., F.R.T.C., P.F.S., R.B., A.S.N., and M.S.D. did analysis and interpretation of data. M.P.D., F.R.T.C., P.F.S., and M.S.D. drafted the manuscript. P.F.S., T.N.M., A.L.A., B.C.S., F.D.C., T.D.C., L.J.R.F., B.F.C.S., V.G.P., M.C.B., J.C.M.M., O.F.P.S., R.B., and M.S.D. made contributions to literature search and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Appendix
COVID-19 ICU Treatment Protocol
Briefly, the criteria for admission to the ICU included at least one of the following: supplemental oxygen (nasal oxygen catheter >3.0 L/min) to keep pulsed oximetry oxygen saturation (SpO2) levels >94% or the respiratory rate ≤24 bpm; noninvasive ventilation to keep SpO2 >94% or respiratory rate ≤24 bpm; acute respiratory failure treated with IMV; hemodynamic instability or shock, defined as arterial hypotension (systolic blood pressure <90 mm Hg or mean arterial pressure <65 mm Hg), or signs of poor organ or peripheral perfusion (altered consciousness, oliguria, lactate ≥2 mmol/L, among others), with or without the use of vasopressors; sepsis with arterial hypotension, need for vasopressor or lactate ≥4 mmol/L; or septic shock according to Sepsis-3 criteria.
In patients who required endotracheal intubation, the initial ventilatory parameters were the following: mode (pressure-controlled ventilation), tidal volume (6 mL/kg of predicted body weight), positive end-expiratory pressure (PEEP) of 15 cm H2O, respiratory rate of 20–24 per minute to keep the minute volume between 7 and 10 L per minute, driving pressure (plateau pressure [PEEP]) of 15 cm H2O, initial target of SpO2 between 92 and 96%, and end-tidal carbon dioxide (EtCO2) between 30 and 45 mm Hg. An arterial blood gas analysis was collected 1 hour after endotracheal intubation for adjustments of the initial mechanical ventilation parameters. If partial arterial pressure of oxygen to fractional inspired oxygen ratio (PaO2/FiO2) was <200, a recruitment maneuver was performed (with PEEP of 25 cm H2O and driving pressure of 15 cm H2O for 5 min) and PEEP was adjusted to 18 cm H2O. If necessary, PEEP was adjusted to a maximum of 20 cm H2O. If PaO2/FiO2 remained <200, then the patient was placed in a prone position.
Hemodynamic management (fluids, vasopressors, and inotropes) aimed to maintain an average blood pressure of 65 mm Hg and proper tissue perfusion parameters. If at least 2 of the following markers were present (lactate >18 mg/dL, GapCO2 >6 mm Hg, and capillary refill time <3 s), cardiac output was monitored. Invasive blood pressure monitoring by arterial cannulation and a central venous catheter placement were used in patients receiving noradrenaline at a dose >0.1 mcg/kg/min. If noradrenaline dose is <0.5 mcg/kg/min, adrenaline 0.01 mcg/kg/min was started. Hydrocortisone continuous infusion of 200 mg was used if norepinephrine was >0.2 mcg/kg/min at the end of 6 h of resuscitation. After 48 h of IMV, maintenance of null or negative fluid balance was suggested using furosemide, if necessary. If there was an increase in the need for vasopressors or changes in tissue perfusion markers, clinical and laboratory signs suggestive of hypovolemia, null or positive fluid balance was advised.
At that time, the use of hydroxychloroquine at a dose of 400 mg every 12 days and azithromycin 500 mg once daily for 10 days, was recommended. Pantoprazole 40 mg once daily and unfractionated heparin 5,000 IU subcutaneously 3 times daily were used as prophylaxis for stress ulcers and venous thromboembolism, respectively. Ceftriaxone or ceftaroline were used as first-line antibiotic therapy when a bacterial infection was suspect.
References
- 1.Coronavírus. Ministério da Saúde. Brasília, Brasil. https://www.saude.gov.br/ Accessed 2020 Jul 20.
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with covid-19. Kidney Int. 2020;98((1)):209–18. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180((7)):934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382((18)):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain-Syed F, Birk HW, Seeger W, Ronco C. A protective kidney-lung approach to improve outcomes in mechanically ventilated patients. Blood Purif. 2016;42((3)):214–8. doi: 10.1159/000448471. [DOI] [PubMed] [Google Scholar]
- 8.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41((8)):1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97((5)):829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting caracteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323((20)):2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323((16)):1612–4. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31((6)):1157–65. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7((5)):712–9. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2((1)):1–138. [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150((9)):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durão MS, Monte JC, Batista MC, Oliveira M, Iizuka IJ, Santos BF, et al. The use of regional citrate anticoagulation for continuous venovenous hemodiafiltration in acute kidney injury. Crit Care Med. 2008;36((11)):3024–9. doi: 10.1097/CCM.0b013e31818b9100. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing; 2019. [Google Scholar]
- 18.Lim J-H, Park S-H, Jeon Y, Cho J-H, Jung H-Y, Choi J-Y, et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020;9((6)):1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180((11)):1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46((6)):1114–6. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin S, Orieux A, Prevel R, Garric A, Bats ML, Dabernat S, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. 2020;13((3)):354–61. doi: 10.1093/ckj/sfaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fominskiy EV, Scandroglio AM, Monti G, Calabrò MG, Landoni G, Dell'Acqua A, et al. Prevalence, characteristics, risk factors, and outcomes of invasively ventilated COVID-19 patients with acute kidney injury and renal replacement therapy. Blood Purif. 2020:1–8. doi: 10.1159/000508657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013;17((3)):R98–2. doi: 10.1186/cc12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darmon M, Clec'h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E, et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol. 2014;9((8)):1347–53. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyner JL, Murray PT. Mechanical ventilation and the kidney. Blood Purif. 2010;29((1)):52–68. doi: 10.1159/000259585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panitchote A, Mehkri O, Hastings A, Hanane T, Demirjian S, Torbic H, et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019;9((1)):74. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta RL, Pascual MT, Soroko S, Chertow GM. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288((20)):2547–53. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 28.Wiedemann HP, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354((24)):2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. 2010;5((9)):1690–5. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 30.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396((10247)):320–32. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395((10239)):1763–70. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murashima M, Nishimoto M, Kokubu M, Hamano T, Matsui M, Eriguchi M, et al. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci Rep. 2019;9((1)):20260–9. doi: 10.1038/s41598-019-56615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y, Huang XR, Lv J, Chung AC, Zhang Y, Chen JZ, et al. C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin Sci. 2014;126((9)):645–59. doi: 10.1042/CS20130471. [DOI] [PubMed] [Google Scholar]
- 34.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansrivijit P, Qian C, Boonpheng B, Thongprayoon C, Vallabhajosyula S, Cheungpasitporn W, et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. 2020;68((7)):1261–70. doi: 10.1136/jim-2020-001407. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Jin Y, Li R, Zhang Z, Sun R, Chen D. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24((1)):356. doi: 10.1186/s13054-020-03065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180((10)):1345–55. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y, Luo R, Wang X, Wang K, Zhang N, Zhang M, et al. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15((10)):1394–402. doi: 10.2215/CJN.04650420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007 Sep;72((5)):624–31. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 40.Elhmidi Y, Bleiziffer S, Piazza N, Hutter A, Opitz A, Hettich I, et al. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J. 2011;161((4)):735–9. doi: 10.1016/j.ahj.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Dos Santos RP, Carvalho ARDS, Peres LAB. Incidence and risk factors of acute kidney injury in critically ill patients from a single centre in Brazil: a retrospective cohort analysis. Sci Rep. 2019;9((1)):18141. doi: 10.1038/s41598-019-54674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y, Nie S, Li L, Li Y, Liu D, Xiong M, et al. EACH study investigators. Epidemiology and outcomes of acute kidney injury in hospitalized cancer patients in China. Int J Cancer. 2019;144((11)):2644–50. doi: 10.1002/ijc.31993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data