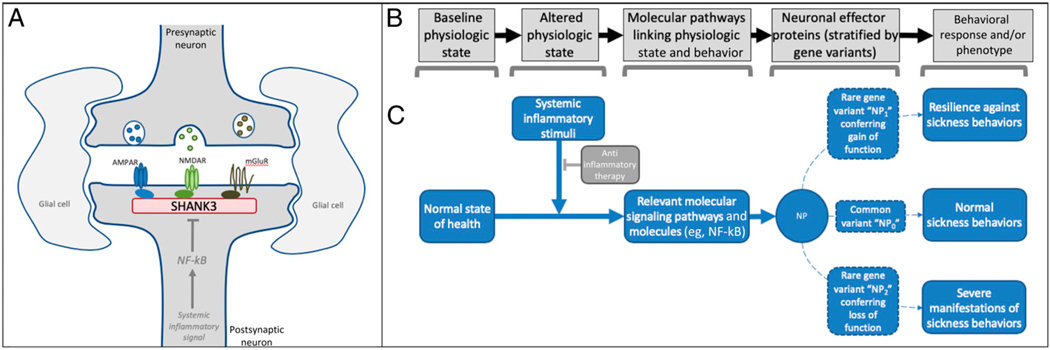
FIGURE 1.
Sickness behavior as a hypothetical model for conceptualizing SHANK3’s interface with the immune system. SHANK3 functions as an important scaffolding protein for neurotransmitter receptors (see 1). A, In experimental models, systemic inflammatory stimuli can decrease SHANK3 expression via nuclear factor κ–light-chain enhancer of activated B cells (NF-kB)-related signaling pathways (see 8). B, A variety of stereotyped behaviors occur as codified responses to nonhomeostatic physiologic states (eg, acute illness), each using unique molecular pathways to manifest adaptive, stimuli-specific behavioral responses. C, Alteration of proteins within these pathways, such as via genetic variants that confer a gain or loss of function, would be hypothesized to alter the associated behavioral response. The diverse behaviors that accompany states of acute infection, known as sickness behaviors, which include irritability, depressed mood, and social withdrawal, offer a template for conceptualizing the molecular and behavioral phenomena that may result from systemic inflammation and, conversely, be modified by its resolution (see 17). Considered together with our case histories, the combined observations offer a reasonable hypothesis implicating SHANK3 as a candidate protein involved in 1 or more molecular pathways regulating clinically relevant sickness behaviors in humans. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; mGluR, metabotropic glutamate receptor; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NMDAR, N-methyl-D-aspartate receptor; NP0, common variant of a hypothetical neurobehavioral protein; NP1, gain of function variant of neurobehavioral protein; NP2, loss of function of neurobehavioral protein; NP, neurobehavioral protein.