Figure 1.
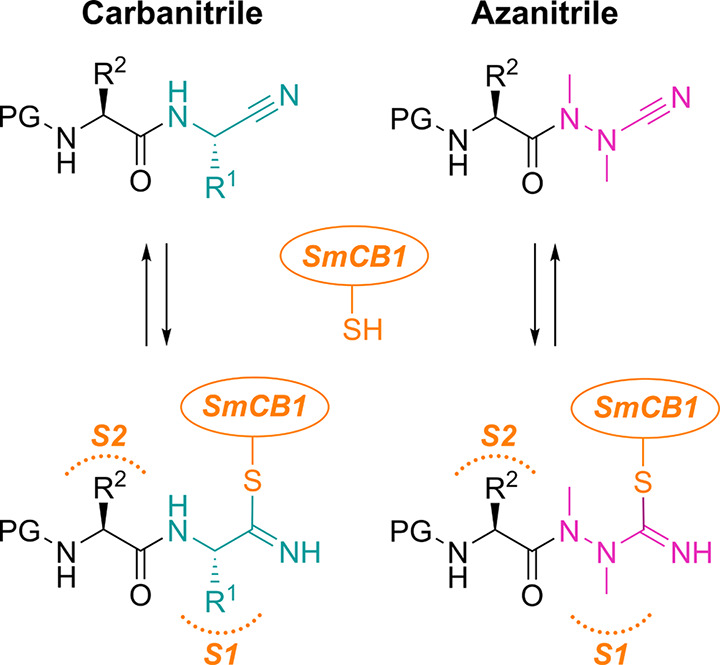
Carba- and azadipeptide nitriles and their reaction with cysteine proteases. Isoelectronic CαH/N exchange in the warhead (cyan/magenta) of dipeptide nitriles (left) leads to azadipeptide nitriles (right). Two carbohydrazide nitrogens in azanitriles need to be alkylated to circumvent spontaneous heterocyclization.6 R1 and R2 are substituents in amino acid residues at the P1 and P2 positions (binding in the enzyme subsites S1 and S2), respectively; PG is a protecting group. The depicted azadipeptide bears an aza-alanine nitrile at the P1 position. Reactive warheads of both chemotypes form a covalent, reversible bond with the thiol of the catalytic cysteine residue (orange) of papain-family cysteine proteases (represented by SmCB1).
