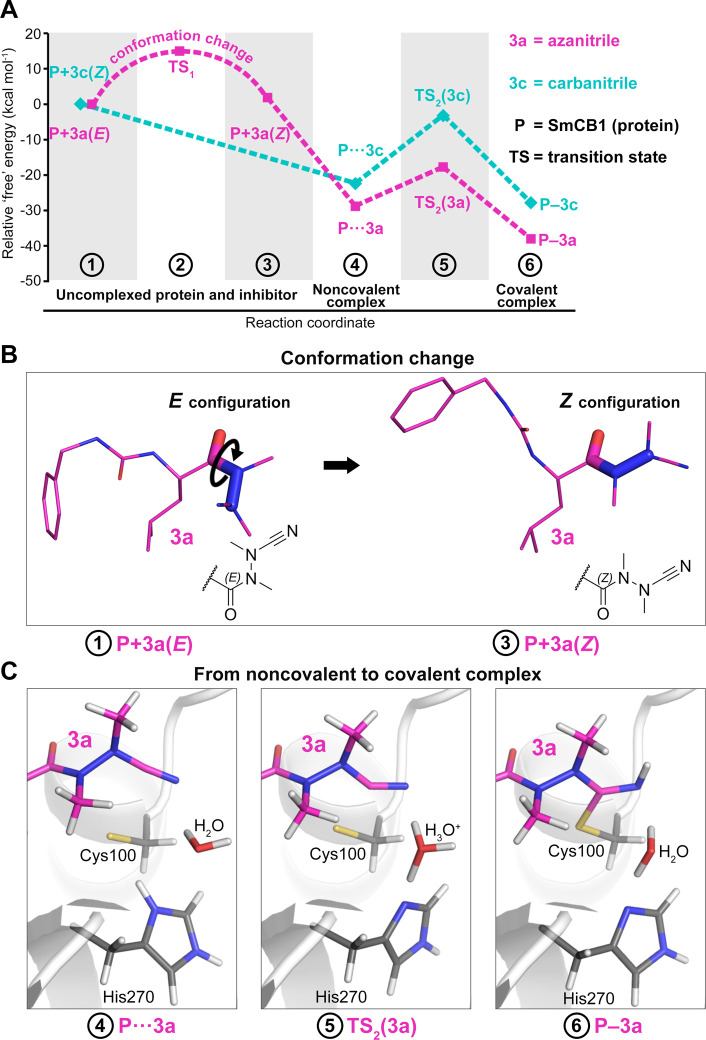
Figure 5.
Computational analysis of the binding reaction of azanitrile and carbanitrile inhibitors to the active site of SmCB1. (A) The “free” energy profile of the binding of azanitrile 3a and carbanitrile 3c was determined using quantum chemical calculations. Individual states along the reaction pathway (indicated by numbers) are defined by their relative “free” energies (Table S6). (B) The unbound azanitrile inhibitor has the E-configuration in solution (with minimum “free” energy) and undergoes a conformational change to the Z-configuration that was also demonstrated crystallographically in the SmCB1–3a complex. (C) Modeled states upon binding of the azanitrile inhibitor to the active site include an initial noncovalent complex (4), a transition state with proton transfer from His270 to H2O (5), and a final covalent complex after proton transfer to the nitrile group (6). The distance (sulfur–carbon) of the catalytic Cys100 and the inhibitor’s CAB atom is 3.2, 2.3, and 1.8 Å, respectively.