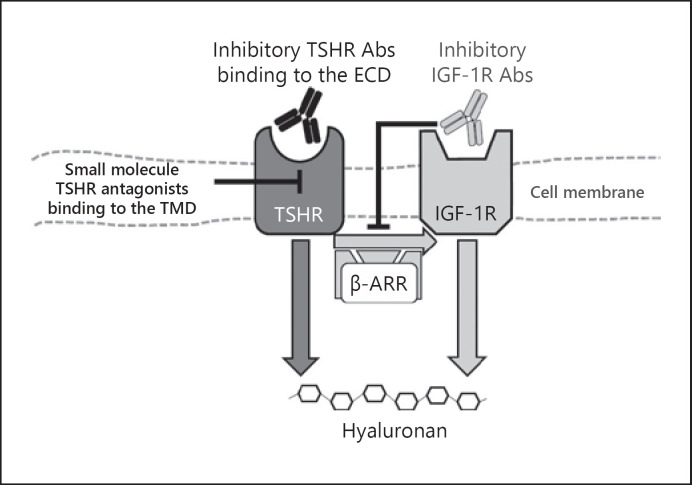
Fig. 4.
Model of inhibition of TSHR/IGF-1R crosstalk by targeting TSHR and/or IGF-1R. Activation of the TSHR by GO-Igs stimulates two signal transduction pathways − one that is independent of IGF-1R (dark gray arrow) and another that is dependent on IGF-1R, which is the crosstalk pathway (light gray arrows). The activated TSHR engages the IGF-1R in a signalosome that is scaffolded by β-arrestin 1 (β-ARR), and this crosstalk induces the synergistic increase in HA secretion. As TSHR and IGF-1R signal in concert in the pathogenesis of TED, therapeutic interventions that target both receptors may be desirable. In contrast to antigen-specific immunotherapies which use peptides of sequences in the extracellular domain of the TSHR, inhibitory TSHR antibodies (Abs) and small molecule TSHR antagonists directly target the TSHR and inhibit signaling. TSHR-blocking antibodies inhibit TSHR activation by binding to the extracelluar domain (ECD) of the TSHR thereby inhibiting GO-Igs from binding. Drug-like, small molecule TSHR antagonists bind to allosteric sites in the transmembrane domain (TMD) of TSHR and inhibit activation by hindering signal transduction through the TSHR from the extracellular to the intracellular domain which subsequently inhibits interaction of the receptor with G proteins and other intracellular signaling molecules. IGF-1R-inhibiting antibodies, like 1H7 and teprotumumab, bind to IGF-1R and inhibit IGF-1R crosstalk with TSHR. It is conceivable that a combination therapy with TSHR and IGF-1R antagonists may have therapeutic benefits as it could minimize drug side effects due to dose reduction and may compensate for any loss of anti-IGF-1R efficacy.