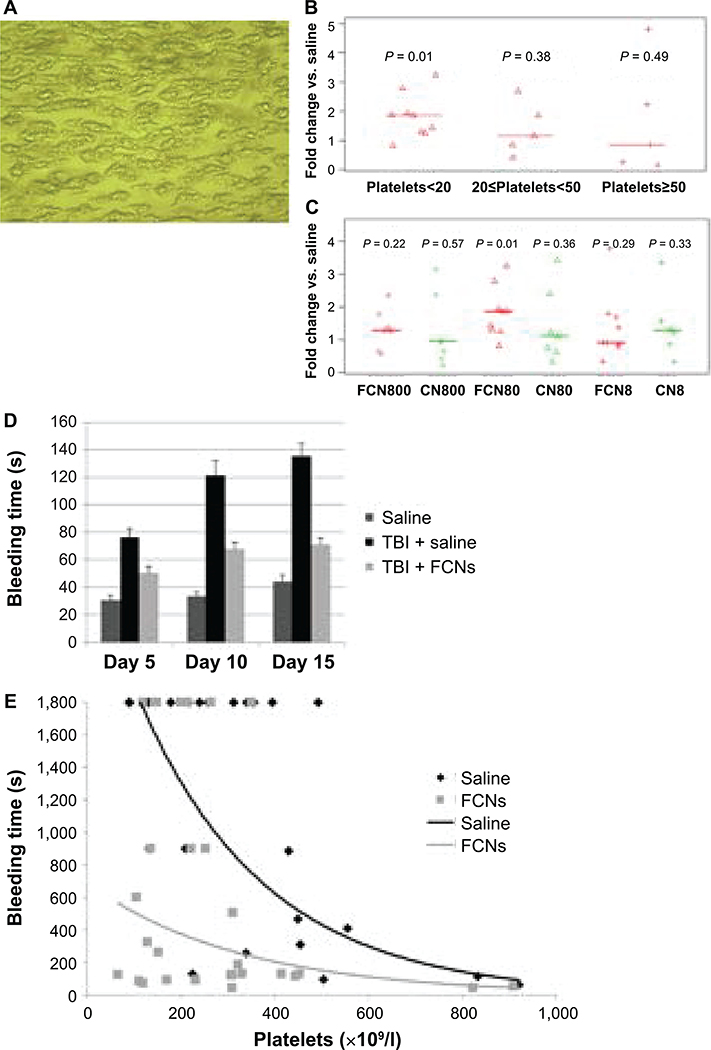
FIG. 2.
FCNs improve bleeding time by enhancing platelet aggregation. We observed the effects of FCNs under flow-based conditions (see Materials and Methods). FCNs, control nanospheres (CNs) or saline were added to whole blood samples from patients with varying degrees of thrombocytopenia. Images were taken of platelets adhered to a collagen-coated chip (panel A) and analyzed to determine the total area of platelet aggregation (40× magnification). A scale bar was also made. No significant difference was observed in the area of aggregation when FCNs were added to samples from patients whose platelet counts were greater than 20,000/μl; however, a twofold increase in aggregation was observed when FCNs were added to samples from patients whose platelet counts were <20,000/μl (panel B). We further explored these findings by comparing FCNs, 80 μg/ml, to tenfold-higher and -lower doses, as well as to the corresponding doses of CNs. (FCN800=FCNs 800 μg/ml; FCN80=FCNs 80 μg/ml; FCN8=FCNs 8 μg/ml; CNs=plain albumin without fibrinogen coating; CN800=CNs 800 μg/ml; CN80=CNs 80 μg/ml; and CN8=CNs 8 μg/ml). FCNs, 80 μg/ml, appeared to be the most efficacious, whereas the other results were varied and inconsistent (panel C). In a tail vein bleeding model, the bleeding time was significantly shorter in mice treated with FCNs than those treated with saline at days 5, 10 and 15 postirradiation (panel D). A saphenous vein bleeding model was also used, in which mice were given anti-CD42b anti-platelet antibody to induce varying degrees of thrombocytopenia and treated with saline or FCNs before saphenous vein injury. Time to cessation of bleed was measured, with bleeding time capped at 30 min. Bleeding time was significantly shortened in the mice receiving FCNs, particularly when the platelet count was <200,000/μl (P = 0.030) or 200–400,000/μl (P = 0.046). (panel E).