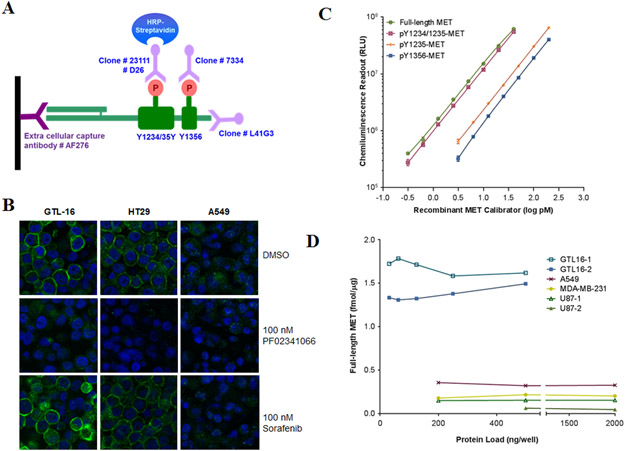
Figure 1. Development of immunoassays for full-length MET and its key phosphorylated species.
(A) Schematic of the MET immunoassays: a capture antibody (catalog number AF276) specific to the extracellular domain of MET binds and traps MET from tissue lysates, then four different reporter monoclonal antibodies (clone D26 to pY1234/1235, clone 23111 to pY1235, clone 7334 to pY1356 and clone L41G3 to the C-terminus) are used to detect phosphorylated and full-length MET. (B) Specificity of anti-pY1235-MET (clone 23111; developed in this study) is shown by immunofluorescence staining of formalin-fixed, paraffin-embedded GTL-16, HT29, and A549 cancer cells treated in vitro with 100 nM PF02341066 or 100 nM sorafenib for 4 h. (C) Representative calibration curves from the full-length MET (0.3-40 pM), pY1234/1235-MET (0.3-40 pM), pY1235-MET (1.5-200 pM), and pY1356-MET immunoassays (3.25-200 pM). The same rMET protein is used as a calibrator in all four immunoassays and levels have been converted to log pM. (D) MET levels in four different human cancer cell lines routinely used in MET preclinical xenograft studies; two separate GTL-16 and U87 xenograft samples were used. Cell lysates were diluted to 31.25-2000 ng/well in a 100 μL volume; MET levels were back-calculated to fmol/μg total loaded protein.