Figure 9.
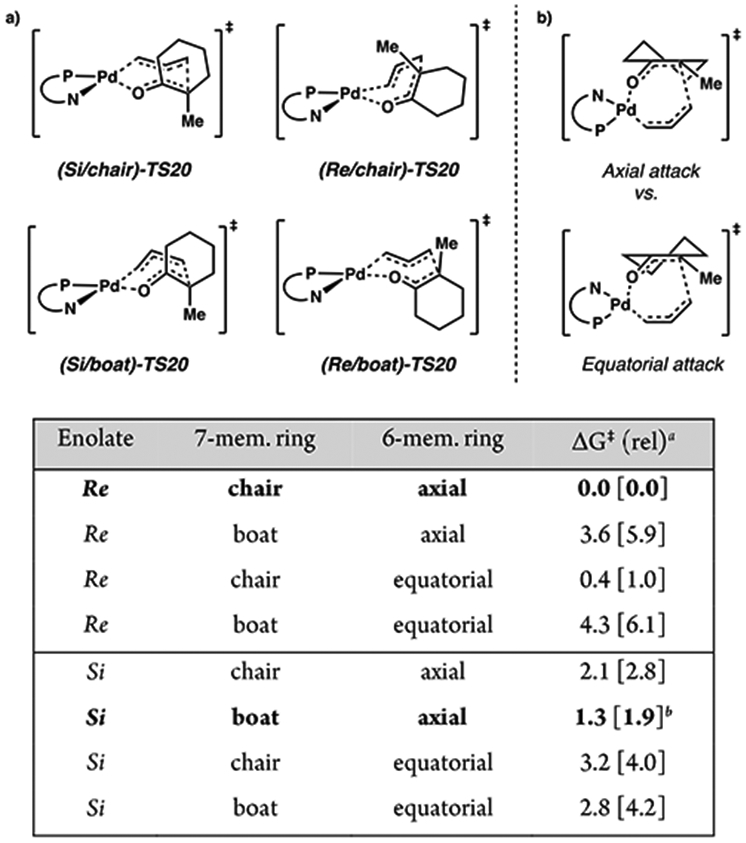
(a) Four conformational geometries of the seven-membered ring (top). (b) Two cyclic enolate half-chair conformers possible for each of the four conformers depicted in part a. Comparison of relative free energy barrier heights among eight 7-membered ring reductive elimination transition states ( bottom; TS20). aRelative free energies given in kcal/mol at the M06/def2-TZVP–CPCM(THF) level of theory with DLPNO-CCSD(T)/cc-pVTZ–CPCM(THF) values in brackets. bWith TightPNO settings.
